The risk tolerance and decision-making processes of Australian women regarding medication trials in pregnancy
- PMID: 39333018
- PMCID: PMC12099191
- DOI: 10.1111/ajo.13884
The risk tolerance and decision-making processes of Australian women regarding medication trials in pregnancy
Abstract
Background: Pregnant women have historically been excluded from participation in medication trials, in part due to the perceived risks of drug exposure to mothers and fetuses. However, little is known about pregnant women's attitudes toward risk and participation in such trials.
Aims: To address this knowledge gap and to identify factors that influence trial participation.
Materials and methods: Australian women over the age of 18, currently pregnant or within six months of delivery, were recruited to participate in an online survey (n = 623) and follow-up interviews (n = 11). The survey investigated willingness to participate in five hypothetical drug trial scenarios of varying risk. Demographic and obstetric information, including COVID-19 vaccination status, was also collected. The impact of these factors on trial participation was analysed using ordinal regression. Interviews were subjected to thematic framework analysis using a priori and emergent themes.
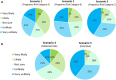
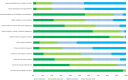
Results: Nearly half of the respondents (48%) indicated a willingness to participate in at least one of the hypothetical trials. As trial risk increased participation likelihood decreased, especially if the risk was to the fetus, regardless of benefits to the mother. COVID-19 vaccination status and medication hesitancy were predictors of an unwillingness to participate. Three broad themes emerged from the qualitative data: risk-benefit analysis, quality of evidence, and trust.
Conclusions: Overall, participants expressed a positive attitude toward research and medication trials during pregnancy, but were concerned about fetal risk. The findings of this study may help enhance trial design and the participation of pregnant women in medication trials.
Keywords: clinical trial; fetus; mothers; pregnancy; trust.
© 2024 The Author(s). Australian and New Zealand Journal of Obstetrics and Gynaecology published by John Wiley & Sons Australia, Ltd on behalf of Royal Australian and New Zealand College of Obstetricians and Gynaecologists.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- National Institutes of Health . What Are Clinical Trials and Studies? Vol. 2022. Bethesda, MD: National Institutes of Health, 2022.
-
- Scaffidi J, Mol BW, Keelan JA. The pregnant women as a drug orphan: A global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG 2017; 124: 132–140. - PubMed
-
- Herring C, McManus A, Weeks A. Off‐label prescribing during pregnancy in the UK: An analysis of 18,000 prescriptions in Liverpool Women's hospital. Int J Pharm Pract 2010; 18: 226–229. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

