Selective regulation of macrophage lipid metabolism via nanomaterials' surface chemistry
- PMID: 39333092
- PMCID: PMC11436645
- DOI: 10.1038/s41467-024-52609-7
Selective regulation of macrophage lipid metabolism via nanomaterials' surface chemistry
Abstract
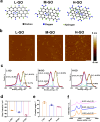
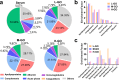
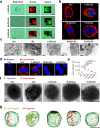
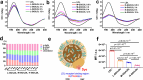
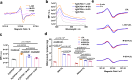
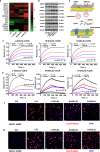
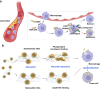
Understanding the interface between nanomaterials and lipoproteins is crucial for gaining insights into their impact on lipoprotein structure and lipid metabolism. Here, we use graphene oxide (GOs) nanosheets as a controlled carbon nanomaterial model to study how surface properties influence lipoprotein corona formation and show that GOs have strong binding affinity with low-density lipoprotein (LDL). We use advanced techniques including X-ray reflectivity, circular dichroism, and molecular simulations to explore the interfacial interactions between GOs and LDL. Specifically, hydrophobic GOs preferentially associate with LDL's lipid components, whereas hydrophilic GOs tend to bind with apolipoproteins. Furthermore, these GOs distinctly modulate a variety of lipid metabolism pathways, including LDL recognition, uptake, hydrolysis, efflux, and lipid droplet formation. This study underscores the importance of structure analysis at the nano-biomolecule interface, emphasizing how nanomaterials' surface properties critically influence cellular lipid metabolism. These insights will inspire the design and application of future biocompatible nanomaterials and nanomedicines.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- 22388101, 22027810/National Natural Science Foundation of China (National Science Foundation of China)
- IS23023/Natural Science Foundation of Beijing Municipality (Beijing Natural Science Foundation)
- IS23023/Natural Science Foundation of Beijing Municipality (Beijing Natural Science Foundation)
- 2023M742048/Postdoctoral Research Foundation of China (China Postdoctoral Research Foundation)
LinkOut - more resources
Full Text Sources

