Crosslinking of Ly6a metabolically reprograms CD8 T cells for cancer immunotherapy
- PMID: 39333093
- PMCID: PMC11437002
- DOI: 10.1038/s41467-024-52079-x
Crosslinking of Ly6a metabolically reprograms CD8 T cells for cancer immunotherapy
Abstract
T cell inhibitory mechanisms prevent autoimmune reactions, while cancer immunotherapy aims to remove these inhibitory signals. Chronic ultraviolet (UV) exposure attenuates autoimmunity through promotion of poorly understood immune-suppressive mechanisms. Here we show that mice with subcutaneous melanoma are not responsive to anti-PD1 immunotherapy following chronic UV irradiation, given prior to tumor injection, due to the suppression of T cell killing ability in skin-draining lymph nodes. Using mass cytometry and single-cell RNA-sequencing analyzes, we discover that skin-specific, UV-induced suppression of T-cells killing activity is mediated by upregulation of a Ly6ahigh T-cell subpopulation. Independently of the UV effect, Ly6ahigh T cells are induced by chronic type-1 interferon in the tumor microenvironment. Treatment with an anti-Ly6a antibody enhances the anti-tumoral cytotoxic activity of T cells and reprograms their mitochondrial metabolism via the Erk/cMyc axis. Treatment with an anti-Ly6a antibody inhibits tumor growth in mice resistant to anti-PD1 therapy. Applying our findings in humans could lead to an immunotherapy treatment for patients with resistance to existing treatments.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
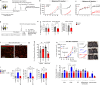




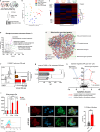
References
-
- Romerdahl, C. A., Donawho, C., Fidler, I. J. & Kripke, M. L. Effect of ultraviolet-B radiation on the in vivo growth of murine melanoma cells. Cancer Res48, 4007–4010 (1988). - PubMed
-
- Donawho, C. K. & Kripke, M. L. Evidence that the local effect of ultraviolet radiation on the growth of murine melanomas is immunologically mediated. Cancer Res51, 4176–4181 (1991). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

