Neuronal alpha-Synuclein Disease integrated staging system performance in PPMI, PASADENA, and SPARK baseline cohorts
- PMID: 39333167
- PMCID: PMC11567150
- DOI: 10.1038/s41531-024-00789-w
Neuronal alpha-Synuclein Disease integrated staging system performance in PPMI, PASADENA, and SPARK baseline cohorts
Abstract
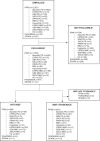
The Neuronal alpha-Synuclein Disease (NSD) biological definition and Integrated Staging System (NSD-ISS) provide a research framework to identify individuals with Lewy body pathology and stage them based on underlying biology and increasing degree of functional impairment. Utilizing data from the PPMI, PASADENA, and SPARK studies, we developed and applied biologic and clinical data-informed definitions for the NSD-ISS across the disease continuum. Individuals enrolled as Parkinson's disease, Prodromal, or Healthy Controls were defined and staged based on biological, clinical, and functional anchors at baseline. Across the three studies 1741 participants had SAA data and of these 1030 (59%) were S+ consistent with NSD. Among sporadic PD, 683/736 (93%) were NSD, and the distribution for Stages 2B, 3, and 4 was 25%, 63%, and 9%, respectively. Median (95% CI) time to developing a clinically meaningful outcome was 8.3 (6.2, 10.1), 5.9 (4.1, 6.0), and 2.4 (1.0, 4.0) years for baseline stage 2B, 3, and 4, respectively. We propose pilot biologic and clinical anchors for NSD-ISS. Our results highlight the baseline heterogeneity of individuals currently defined as early PD. Baseline stage predicts time to progression to clinically meaningful milestones. Further research on validation of the anchors in longitudinal cohorts is necessary.
© 2024. The Author(s).
Conflict of interest statement
T.D. declares former employment for and employee stock options in Biogen. G.P. declares employment for F. Hoffmann-La Roche Ltd. and stock ownership for F. Hoffmann-La Roche Ltd., Atea, Novartis and Eli Lilly. M.B. declares travel grants from The Michael J. Fox Foundation. C.G. declares employment for The Michael J. Fox Foundation. KP declares consultancies for Curasen; was on a scientific advisory board for Curasen and Amprion; honoraria from invited scientific presentations to universities and professional societies not exceeding $5000 per year from California Congress of Clinical Neurology, California Neurological Society, and Johns Hopkins University; and patents or patent applications numbers 17/314,979 and 63/377,293. K.P. also declares grants to her institution (Stanford University School of Medicine) from NIH/NINDS NS115114, NS062684, NS075097, NIH/NIA U19 AG065156, P30 AG066515, The Michael J. Fox Foundation, Lewy Body Dementia Association, Alzheimer’s Drug Discovery Foundation, Sue Berghoff LBD Research Fellowship and the Knight Initiative for Brain Resilience. DW declares salary support from The Michael J. Fox Foundation for serving on an Executive Steering Committee for the PPMI and consultancies for Roche Pharma. D.W. declares membership on the npj P.D. Editorial Board. L.C. declares grants to her institution from Biogen (clinical trial funding), MJFF, UPMC Competitive Medical Research Fund, National Institutes of Health, and University of Pittsburgh; grant and travel support from MJFF; royalties from Wolters Kluwel (for authorship); and in-kind donation by Advanced Brain Monitoring of equipment for research study to her institution. CC declares grants from The Michael J. Fox Foundation and NIH/NINDS. CT declares consultancies for CNS Ratings, Australian Parkinson’s Mission, Biogen, Evidera, Cadent (data safety monitoring board), Adamas (steering committee), Biogen (via the Parkinson Study Group steering committee), Kyowa Kirin (advisory board), Lundbeck (advisory board), Jazz/Cavion (steering committee), Acorda (advisory board), Bial (DMC) and Genentech. CT also declares grant support to her institution from The Michael J. Fox Foundation, National Institute of Health, Gateway LLC, Department of Defense, Roche Genentech, Biogen, Parkinson Foundation and Marcus Program in Precision Medicine. C.T. declares membership on the npj PD Editorial Board. C.K. declares employment for The Michael J. Fox Foundation. Y.X. declares employment for and travel grants from The Michael J. Fox Foundation. SC declares employment for and travel grants from The Michael J. Fox Foundation. LC-M declares employment for and employee stock options in Amprion, grants 16712 and 21233 from The Michael J. Fox Foundation to his institution; grant U44NS111672 from NIH to his institution; and patents or patent application numbers US 20210277076A1, US 20210311077A1, US 20190353669A1 and US 20210223268A1. P.D. has no declarations. TF declares travel grants and grant payments to her institution (Indiana University) from The Michael J. Fox Foundation. M.F. declares employment for The Michael J. Fox Foundation and an unpaid advisory role at Vaxxinity. D.J. declares employment for and employee stock options from Denali Therapeutics. KK declares support to his institution (University of Rochester Medical Center) from The Michael J. Fox Foundation. KMe declares consultancies for The Michael J. Fox Foundation, AcuRx, Caraway, Cerebral Therapeutics, NRG Therapeutics (scientific advisory board), Nitrase Therapeutics (scientific advisory board), Nurabio, Retromer Therapeutics (director on the board, part-time chief scientific officer), Schrodinger, Sinopia Biosciences (scientific advisory board), and Vanqua Biosciences (scientific advisory board); stock ownership for Cognition Therapeutics, Eli Lilly (retiree stock holder), Envisagenics, Nitrase Therapeutics, Sinopia Biosciences and Retromer Therapeutics; honoraria for the University of Utah; patents or patent applications for Retromer Therapeutics (planned patent); research grant from The Michael J. Fox Foundation and travel grants from the University of Utah. BM declares consultancies from Roche and Biogen; grants from The Michael J. Fox Foundation, A.S.A.P. and D.F.G.; honoraria for Abbvie and Bial; leadership role for The Michael J. Fox Foundation and travel grants for Abbvie. T.M. declares support to his institution (Stanford University School of Medicine) from The Michael J. Fox Foundation. K.N. declares grant to her institution from The Michael J. Fox Foundation. JS declares consultancies from Invicro, Biogen, and Abbvie; and stock ownership from RealmIDX, MNI Holdings, and LikeMinds as well as grants from The Michael J. Fox Foundation. TSh declares employment for The Michael J. Fox Foundation. ASin declares employment for The National Institutes of Health who received grants from The Michael J. Fox Foundation and A.S.A.P. A.S.i.n. declares Diagnostic for Stroke royalties (unrelated to current work); honoraria from Movement Disorders Society and Nature Publishing Group; travel grants from Chan Zuckerberg Initiative, The Michael J. Fox Foundation and Weill Cornell. ASin’s spouse is an employee of GeneDx. ASin declares being an associate editor for the npj PD Editorial Board. DS has no declarations. MS declared consultancies for Mediflix, Inc., Health and Wellness Partners; honoraria from Atria Foundation, International Parkinson and Movement Disorder Society, Neurocrine, Luye Pharma, and Acorda. MS serves on advisory board at Neuroderm, Alexza, Alexion, and Biogen. CS declares employment for Amprion; stock ownership for Amprion; honoraria (will receive royalties for the sale of seed amplification assay [SAA]) from Amprion; and patents or patent applications, awarded and amplified in conjunction with Amprion for the SAA assay. ET has no declarations. ASid declares consultancies for SPARC Therapeutics, Capsida Therapeutics, and Parkinson Study Group; honoraria from Bial; grants from The Michael J. Fox Foundation (member of PPMI Steering Committee); and participation on board at Wave Life Sciences, Inhibikase, Prevail, Huntington Study Group and Massachusetts General Hospital. BD declarations are T.B.D. TSi declares consultancies for AcureX, Adamas, AskBio, Amneal, Blue Rock Therapeutics, Critical Path for Parkinson’s Consortium, Denali, The Michael J. Fox Foundation, Neuroderm, Roche, Sanofi, Sinopia, Takeda, and Vanqua Bio; on advisory boards for AcureX, Adamas, AskBio, Biohaven, Denali, GAIN, Neuron23 and Roche; on scientific advisory boards for Koneksa, Neuroderm, Sanofi and U.C.B.; and received research funding from Amneal, Biogen, Roche, Neuroderm, Sanofi, Prevail and UCB and an investigator for NINDS, MJFF, Parkinson’s Foundation. KMa declares support to his institution (Institute for Neurodegenerative Disorders) from The Michael J. Fox Foundation. K.M.a., also declares consultancies for Invicro, The Michael J. Fox Foundation, Roche, Calico, Coave, Neuron23, Orbimed, Biohaven, Anofi, Koneksa, Merck, Lilly, Inhibikase, Neuramedy, IRLabs and Prothena and participates on DSMB at Biohaven.
Figures

Update of
-
Neuronal alpha-Synuclein Disease Integrated Staging System performance in PPMI, PASADENA, and SPARK baseline cohorts.medRxiv [Preprint]. 2024 Sep 10:2024.02.14.24302818. doi: 10.1101/2024.02.14.24302818. medRxiv. 2024. Update in: NPJ Parkinsons Dis. 2024 Sep 27;10(1):178. doi: 10.1038/s41531-024-00789-w. PMID: 39314957 Free PMC article. Updated. Preprint.

