Population pharmacokinetics and exposure-response relationships of maribavir in transplant recipients with cytomegalovirus infection
- PMID: 39333337
- PMCID: PMC11579209
- DOI: 10.1007/s10928-024-09939-2
Population pharmacokinetics and exposure-response relationships of maribavir in transplant recipients with cytomegalovirus infection
Abstract
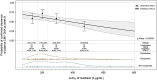
Maribavir is approved for management of post-transplant cytomegalovirus (CMV) infections refractory and/or resistant to CMV therapies at a dose of 400 mg twice daily (BID). Population pharmacokinetic (PopPK) and exposure-response analyses were conducted to support the appropriateness of 400 mg BID dosing. A PopPK model was developed using non-linear mixed-effects modeling with pooled maribavir plasma concentration-time data from phase 1 and 2 studies (from 100 mg up to 1200 mg as single or repeated doses) and the phase 3 SOLSTICE study (400 mg BID). Exposure-response analyses were performed for efficacy, safety, and viral resistance based on data collected in the SOLSTICE study. Maribavir PK after oral administration was adequately described by a two-compartment model with first-order elimination, first-order absorption, and an absorption lag-time. There was no evidence that maribavir PK was affected by age, sex, race, diarrhea, vomiting, disease characteristics, or concomitant use of histamine H2 blockers, or proton pump inhibitors. In the SOLSTICE study, higher maribavir exposure was not associated with increased probability of achieving CMV DNA viremia clearance, nor with reduced probability of treatment-emergent maribavir-resistant CMV mutations. A statistically significant association with maribavir exposure was identified for taste disturbance, fatigue, and treatment-emergent serious adverse events, while transplant type, enrollment region, CMV DNA level at baseline, and/or CMV resistance at baseline were identified as additional risk factors for these safety outcomes. In conclusion, the findings of these PopPK and exposure-response analyses provide further support for the recommended maribavir dose of 400 mg BID.
Keywords: Cytomegalovirus; Exposure response; Maribavir; Population pharmacokinetics; Transplant.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: IH Song, G Chen, and K Sun are employees of Takeda Development Center Americas, Inc., and receive stocks (and stock options) from Takeda. S Hayes and C Farrell are employees of ICON plc. C Jomphe and NH Gosselin are employees of Certara.
Figures




References
-
- Pierce B, Richardson CL, Lacloche L, Allen A, Ison MG (2018) Safety and efficacy of foscarnet for the management of ganciclovir-resistant or refractory cytomegalovirus infections: a single-center study. Transpl Infect Dis 20:e12852. 10.1111/tid.12852 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

