Inhibition of hepatic oxalate overproduction ameliorates metabolic dysfunction-associated steatohepatitis
- PMID: 39333384
- PMCID: PMC11495999
- DOI: 10.1038/s42255-024-01134-4
Inhibition of hepatic oxalate overproduction ameliorates metabolic dysfunction-associated steatohepatitis
Abstract
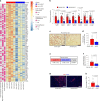
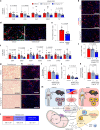
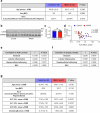
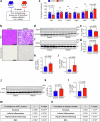
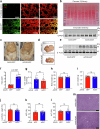
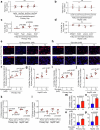
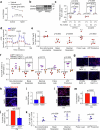
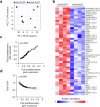
The incidence of metabolic dysfunction-associated steatohepatitis (MASH) is on the rise, and with limited pharmacological therapy available, identification of new metabolic targets is urgently needed. Oxalate is a terminal metabolite produced from glyoxylate by hepatic lactate dehydrogenase (LDHA). The liver-specific alanine-glyoxylate aminotransferase (AGXT) detoxifies glyoxylate, preventing oxalate accumulation. Here we show that AGXT is suppressed and LDHA is activated in livers from patients and mice with MASH, leading to oxalate overproduction. In turn, oxalate promotes steatosis in hepatocytes by inhibiting peroxisome proliferator-activated receptor-α (PPARα) transcription and fatty acid β-oxidation and induces monocyte chemotaxis via C-C motif chemokine ligand 2. In male mice with diet-induced MASH, targeting oxalate overproduction through hepatocyte-specific AGXT overexpression or pharmacological inhibition of LDHA potently lowers steatohepatitis and fibrosis by inducing PPARα-driven fatty acid β-oxidation and suppressing monocyte chemotaxis, nuclear factor-κB and transforming growth factor-β targets. These findings highlight hepatic oxalate overproduction as a target for the treatment of MASH.
© 2024. The Author(s).
Conflict of interest statement
O.R., M.D-G., J.A.G.-V. and F.A. have filed a patent application based on this work: targeting hepatic oxalate overproduction for the treatment of cardiometabolic diseases (PCT/US2024/17626). The other authors declare no competing interests.
Figures










References
-
- Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol.7, 851–861 (2022). - PubMed
-
- Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology67, 328–357 (2018). - PubMed
-
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology142, 711–725 e6 (2012). - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL162294/HL/NHLBI NIH HHS/United States
- R00 HL150233/HL/NHLBI NIH HHS/United States
- R01 DK134011/DK/NIDDK NIH HHS/United States
- HL138139/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL145753/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL141155/HL/NHLBI NIH HHS/United States
- HL159871/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL134569/HL/NHLBI NIH HHS/United States
- R01 DK136685/DK/NIDDK NIH HHS/United States
- HL134569/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL139755/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL153710/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL125838/HL/NHLBI NIH HHS/United States
- R01 HL159871/HL/NHLBI NIH HHS/United States
- P01 AI127335/AI/NIAID NIH HHS/United States
- DK136685/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- HL133497/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL138139/HL/NHLBI NIH HHS/United States
- 24POST1196650/American Heart Association (American Heart Association, Inc.)
- HL141155/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL109946/HL/NHLBI NIH HHS/United States
- P20 GM134974/GM/NIGMS NIH HHS/United States
- K99 HL150233/HL/NHLBI NIH HHS/United States
- HL109946/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL159461/HL/NHLBI NIH HHS/United States
- 19POST34380224/American Heart Association (American Heart Association, Inc.)
- 24POST1199805/American Heart Association (American Heart Association, Inc.)
- DK134011/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 AI056077/AI/NIAID NIH HHS/United States
- 23POST1026505/American Heart Association (American Heart Association, Inc.)
- R01 HL158546/HL/NHLBI NIH HHS/United States
- HL145131/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 20CDA3560123/American Heart Association (American Heart Association, Inc.)
- AI127335/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R00 HL145131/HL/NHLBI NIH HHS/United States
- R01 HL145753/HL/NHLBI NIH HHS/United States
- R01 HL139755/HL/NHLBI NIH HHS/United States
- HL145753-01S1/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL162294/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL150233/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL167758/HL/NHLBI NIH HHS/United States
- K99 HL145131/HL/NHLBI NIH HHS/United States
- HL145753-03S1/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL167758/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL172970/HL/NHLBI NIH HHS/United States
- P20GM134974/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 HL173972/HL/NHLBI NIH HHS/United States
- T32 HL155022/HL/NHLBI NIH HHS/United States
- R56-AI159672/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- DK131859/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R56 AI159672/AI/NIAID NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- F31 DK131859/DK/NIDDK NIH HHS/United States
- R01 HL133497/HL/NHLBI NIH HHS/United States
- R01 HL153710/HL/NHLBI NIH HHS/United States
- I01 BX003415/BX/BLRD VA/United States
- HL158546/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- RP190617/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

