Mutation in the mitochondrial chaperone TRAP1 leads to autism with more severe symptoms in males
- PMID: 39333440
- PMCID: PMC11554806
- DOI: 10.1038/s44321-024-00147-6
Mutation in the mitochondrial chaperone TRAP1 leads to autism with more severe symptoms in males
Abstract
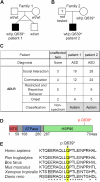
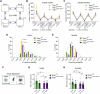
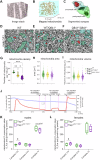
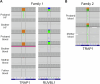
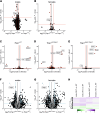
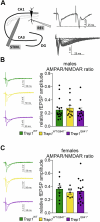
There is increasing evidence of mitochondrial dysfunction in autism spectrum disorders (ASD), but the causal relationships are unclear. In an ASD patient whose identical twin was unaffected, we identified a postzygotic mosaic mutation p.Q639* in the TRAP1 gene, which encodes a mitochondrial chaperone of the HSP90 family. Additional screening of 176 unrelated ASD probands revealed an identical TRAP1 variant in a male patient who had inherited it from a healthy mother. Notably, newly generated knock-in Trap1 p.Q641* mice display ASD-related behavioral abnormalities that are more pronounced in males than in females. Accordingly, Trap1 p.Q641* mutation also resulted in sex-specific changes in synaptic plasticity, the number of presynaptic mitochondria, and mitochondrial respiration. Thus, the TRAP1 p.Q639* mutation is the first example of a monogenic ASD caused by impaired mitochondrial protein homeostasis.
Keywords: Autism; Mitochondria; Mouse Model; Synapses; Trap1.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
-
- Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, Matsuzaki H, Miyachi T, Yamada S, Tsujii M, Tsuchiya KJ, Matsumoto K, Iwata Y, Suzuki K, Ichikawa H, Sugiyama T, Yoshikawa T, Mori N (2012) Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism 3:12 - DOI - PMC - PubMed
-
- Boles RG, Hornung HA, Moody AE, Ortiz TB, Wong SA, Eggington JM, Stanley CM, Gao M, Zhou H, McLaughlin S, Zare AS, Sheldon KM, Skolnick J, McKernan KJ (2015) Hurt, tired and queasy: Specific variants in the ATPase domain of the TRAP1 mitochondrial chaperone are associated with common, chronic “functional” symptomatology including pain, fatigue and gastrointestinal dysmotility. Mitochondrion 23:64–70 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

