Predicting COVID-19 booster immunogenicity against future SARS-CoV-2 variants and the benefits of vaccine updates
- PMID: 39333473
- PMCID: PMC11436652
- DOI: 10.1038/s41467-024-52194-9
Predicting COVID-19 booster immunogenicity against future SARS-CoV-2 variants and the benefits of vaccine updates
Abstract
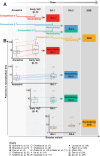
The ongoing evolution of the SARS-CoV-2 virus has led to a move to update vaccine antigens in 2022 and 2023. These updated antigens were chosen and approved based largely on in vitro neutralisation titres against recent SARS-CoV-2 variants. However, unavoidable delays in vaccine manufacture and distribution meant that the updated booster vaccine was no longer well-matched to the circulating SARS-CoV-2 variant by the time of its deployment. Understanding whether the updating of booster vaccine antigens improves immune responses to subsequent SARS-CoV-2 circulating variants is a major priority in justifying future vaccine updates. Here we analyse all available data on the immunogenicity of variants containing SARS-CoV-2 vaccines and their ability to neutralise later circulating SARS-CoV-2 variants. We find that updated booster antigens give a 1.4-fold [95% CI: 1.07-1.82] greater increase in neutralising antibody levels when compared with a historical vaccine immunogen. We then use this to predict the relative protection that can be expected from an updated vaccine even when the circulating variant has evolved away from the updated vaccine immunogen. These findings help inform the rollout of future booster vaccination programmes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Interim safety and immunogenicity of COVID-19 omicron BA.1 variant-containing vaccine in children in the USA: an open-label non-randomised phase 3 trial.Lancet Infect Dis. 2024 Jul;24(7):687-697. doi: 10.1016/S1473-3099(24)00101-4. Epub 2024 Mar 19. Lancet Infect Dis. 2024. PMID: 38518789 Clinical Trial.
-
Immunogenicity and efficacy of XBB.1.5 rS vaccine against the EG.5.1 variant of SARS-CoV-2 in Syrian hamsters.J Virol. 2024 Oct 22;98(10):e0052824. doi: 10.1128/jvi.00528-24. Epub 2024 Sep 4. J Virol. 2024. PMID: 39230305 Free PMC article.
-
Efficacy of parainfluenza virus 5 (PIV5)-vectored intranasal COVID-19 vaccine as a single dose primer and booster against SARS-CoV-2 variants.J Virol. 2025 Apr 15;99(4):e0198924. doi: 10.1128/jvi.01989-24. Epub 2025 Mar 21. J Virol. 2025. PMID: 40116505 Free PMC article.
-
Immunogenicity of a booster dose of a bivalent (Asp614Gly and omicron BA.4/5 variant) self-amplifying mRNA SARS-CoV-2 booster vaccine versus the BNT162b2 omicron BA.4/5 mRNA vaccine: a randomised phase 3 trial.Lancet Infect Dis. 2025 Mar;25(3):290-300. doi: 10.1016/S1473-3099(24)00565-6. Epub 2024 Oct 23. Lancet Infect Dis. 2025. PMID: 39461355 Clinical Trial.
-
Immunogenicity and safety of a bivalent (omicron BA.5 plus ancestral) SARS-CoV-2 recombinant spike protein vaccine as a heterologous booster dose: interim analysis of a phase 3, non-inferiority, randomised, clinical trial.Lancet Infect Dis. 2024 Jun;24(6):581-593. doi: 10.1016/S1473-3099(24)00077-X. Epub 2024 Mar 6. Lancet Infect Dis. 2024. PMID: 38460525 Clinical Trial.
Cited by
-
XBB.1.5 monovalent vaccine induces lasting cross-reactive responses to SARS-CoV-2 variants such as HV.1 and JN.1, as well as SARS-CoV-1, but elicits limited XBB.1.5 specific antibodies.mBio. 2025 Apr 9;16(4):e0360724. doi: 10.1128/mbio.03607-24. Epub 2025 Mar 5. mBio. 2025. PMID: 40042313 Free PMC article.
-
Trans amplifying mRNA vaccine expressing consensus spike elicits broad neutralization of SARS CoV 2 variants.NPJ Vaccines. 2025 Jun 3;10(1):110. doi: 10.1038/s41541-025-01166-1. NPJ Vaccines. 2025. PMID: 40461576 Free PMC article.
-
Predicting pathogen evolution and immune evasion in the age of artificial intelligence.Comput Struct Biotechnol J. 2025 Mar 28;27:1370-1382. doi: 10.1016/j.csbj.2025.03.044. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40235636 Free PMC article. Review.
-
The immunological impact of revaccination in a hybrid-immune world.Front Immunol. 2025 Jun 9;16:1588259. doi: 10.3389/fimmu.2025.1588259. eCollection 2025. Front Immunol. 2025. PMID: 40552302 Free PMC article. Review.
-
RSV F evolution escapes some monoclonal antibodies but does not strongly erode neutralization by human polyclonal sera.J Virol. 2025 Jul 22;99(7):e0053125. doi: 10.1128/jvi.00531-25. Epub 2025 Jul 3. J Virol. 2025. PMID: 40607811 Free PMC article.
References
-
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Changes to Simplify Use of Bivalent mRNA COVID-19 Vaccines (18 April 2023); https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- FDA. FDA Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants (11 September 2023); https://www.fda.gov/news-events/press-announcements/fda-takes-action-upd...
-
- WHO. Statement on the Antigen Composition of COVID-19 vaccines (18 May 2023); https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-compos...
-
- Pfizer. Vaccines and Related Biological Products Advisory Committee June 28, 2022 Meeting Presentation—Pfizer/BioNTech COVID-19 Omicron-Modified Vaccine Options (2022).
-
- Pfizer. Vaccines and Related Biological Products Advisory Committee June 15, 2023 Meeting Presentation- Pfizer: 2023–2024 COVID19 Vaccine Formula—Clinical and Preclinical Supportive Data (2023).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

