Clinical characteristics and influencing factors of anti-PD-1/PD-L1-related severe cardiac adverse event: based on FAERS and TCGA databases
- PMID: 39333574
- PMCID: PMC11436968
- DOI: 10.1038/s41598-024-72864-4
Clinical characteristics and influencing factors of anti-PD-1/PD-L1-related severe cardiac adverse event: based on FAERS and TCGA databases
Abstract
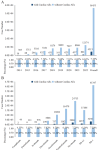
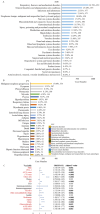
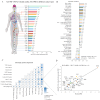
Combining the FDA Adverse Event Reporting System (FAERS) and the Cancer Genome Atlas (TCGA) databases, we aim to explore the factors that influence anti-programmed cell death protein-1 inhibitors/programmed death-ligand-1 (PD-1/PD-L1) related severe cardiac adverse events (cAEs). We obtained anti-PD-1/PD-L1 adverse event reports from January 2014 to December 2022 from the FAERS database. Disproportionality analysis was performed to find anti-PD-1/PD-L1-related cAEs using the proportional reporting ratio (PRR). We were exploring influencing factors based on multivariate logistic regression analysis. Finally, we utilized a strategy that combines FAERS and TCGA databases to explore the potential immune and genetic influencing factors associated with anti-PD-1/PD-L1-related severe cAEs. Reports of severe cAEs accounted for 7.10% of the overall anti-PD-1/PD-L1 adverse event reports in the FAERS database. Immune-mediated myocarditis (PRR = 77.01[59.77-99.23]) shows the strongest toxic signal. The elderly group (65-74: OR = 1.34[1.23-1.47], ≥ 75: OR = 1.64[1.49-1.81]), male (OR = 1.14[1.05-1.24]), anti-PD-L1 agents (OR = 1.17[1.03-1.33]), patients with other adverse events (OR = 2.38[2.17-2.60]), and the concomitant use of proton pump inhibitor (OR = 1.29[1.17-1.43]), nonsteroidal anti-inflammatory drugs (OR = 1.17[1.04-1.31]), or antibiotics (OR = 1.24[1.08-1.43]) may increase the risk of severe cAEs. In addition, PD-L1 mRNA (Rs = 0.71, FDR = 2.30 × 10- 3) and low-density lipoprotein receptor-related protein 3 (LRP3) (Rs = 0.82, FDR = 2.17 × 10- 2) may be immune and genetic influencing factors for severe cAEs. Severe cAEs may be related to antigen receptor-mediated signalling pathways. In this study, we found that age, gender, anti-PD-1/PD-L1 agents, concomitant other adverse events, concomitant medication, PD-L1 mRNA, and LRP3 may be influencing factors for anti-PD-1/PD-L1-related severe cAEs. However, our findings still require a large-scale prospective cohort validation.
Keywords: Anti-PD-1/PD-L1; FAERS; Influence factors; Severe cardiac adverse events; TCGA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086. 10.1158/2159-8290.CD-18-0367 (2018). - DOI - PubMed
-
- Kelley, R. K. et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 401, 1853–1865. 10.1016/S0140-6736(23)00727-4 (2023). - DOI - PubMed
-
- Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 398, 27–40. 10.1016/S0140-6736(21)00797-2 (2021). - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

