The impact of lenvatinib on sarcopenia in patients with advanced unresectable hepatocellular carcinoma
- PMID: 39333610
- PMCID: PMC11437060
- DOI: 10.1038/s41598-024-66766-8
The impact of lenvatinib on sarcopenia in patients with advanced unresectable hepatocellular carcinoma
Abstract
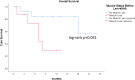
Lenvatinib is a multiple receptor tyrosine kinase inhibitor (TKI) approved for first-line treatment of patients with unresectable hepatocellular carcinoma (HCC). TKI are suspected of exacerbating muscle loss in patients with cancer. In this study, we analyze the role of muscle loss in patients with advanced HCC treated with lenvatinib. This is a retrospective analysis of a real-life cohort of 25 patients with advanced HCC who were treated with lenvatinib from 2018 to March 2021 in Germany. Patients were stratified for loss of skeletal muscle area during the first three months of lenvatinib therapy. Overall survival (OS), progression-free survival (PFS) and toxicity were analyzed for all patients, especially regarding loss of muscle before and during the first three months of therapy with lenvatinib. Three months after beginning of therapy with lenvatinib, a significant reduction of muscle mass was observed in 60% of patients (p = 0.035). Despite increase of loss of skeletal muscle, patients benefitted from lenvatinib in our cohort of patients in terms of OS and PFS and did not experience increased toxicity. Furthermore, muscle loss was not a negative predictor of survival in the univariate analysis (p = 0.675). Patients with advanced hepatocellular carcinoma experience muscle loss with lenvatinib therapy. However, despite progressive muscle loss, patients benefit from a therapy with lenvatinib in terms of OS and PFS without increased toxicity. However, assessment and prophylaxis of skeletal muscle status should be recommended during a therapy with lenvatinib.
© 2024. The Author(s).
Conflict of interest statement
Author MG contributed to advisory boards for Roche, Eisai, MSD, BMS, AZ, Lilly and Servier. However, these activities have no potential conflicts of interest with the manuscript. The other authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

