36-month durability of ultrasound renal denervation for hypertension resistant to combination therapy in RADIANCE-HTN TRIO
- PMID: 39333663
- PMCID: PMC11618087
- DOI: 10.1038/s41440-024-01854-w
36-month durability of ultrasound renal denervation for hypertension resistant to combination therapy in RADIANCE-HTN TRIO
Abstract
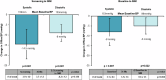
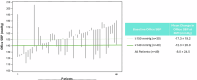
Endovascular ultrasound renal denervation (uRDN) reduced blood pressure (BP) compared to sham at 2 months in patients with resistant hypertension in the multicenter, blinded, randomized, sham-controlled RADIANCE-HTN TRIO trial. This analysis evaluates longer-term outcomes of patients randomized to uRDN. Patients with resistant hypertension to a 3-drug combination pill were randomized to uRDN (n = 69) or sham (n = 67). From 2-5 months, patients followed a standardized anti-hypertensive medication (AHM) titration protocol. At 6 months, patients were unblinded and received AHM per standard of care. In the uRDN group, 71% (49/69) completed 36-month follow-up. Screening office BP was 159/103 on 3.9 AHM. Baseline office BP on the single-pill combination was 153/99 mmHg. At 36 months, office BP changed by -14.5 ± 26.1/-9.0 ± 14.8 mmHg from screening (p < 0.001 for both) and -8.0 ± 24.5/-5.0 ± 14.6 mmHg from baseline (p = 0.007; p = 0.022) on 3.7 AHM. The efficacy of uRDN was durable to 36 months in patients with resistant hypertension with no safety concerns.
Keywords: Hypertension; blood pressure; renal denervation; ultrasound.
© 2024. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: MJB has received personal fees from Recor Medical and Medtronic. AJK reports institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, Recor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, and Merck. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for consulting and/or speaking engagements in which AJK controlled the content. Personal: Consulting from IMDS; Travel Expenses/Meals from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, Recor Medical, Chiesi, OpSens, Zoll, and Regeneron. MA has received research grants from the European Horizon 2020 programme; has received grant support and non-financial support from Recor Medical, Novartis, and Idorsia; and has received personal fees from Alnylam Pharmaceutical, Novartis, Medtronic, Astra Zeneca. FM is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. He has received scientific support from Ablative Solutions, Medtronic and Recor Medical and speaker honoraria/consulting fees from Ablative Solutions, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, Recor Medical, Servier, and Terumo. JB has reported receiving grants from Recor Medical and Ablative Solutions. JD has received institutional grant/research support from Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, Microport, Pie Medical, and Recor Medical, and consultancy and speaker fees from Abbott Vascular, Abiomed, ACIST medical, Boston Scientific, Cardialysis BV, CardiacBooster, Kaminari Medical, Recor Medical, PulseCath, Pie Medical, Sanofi, Siemens Health Care and Medtronic. MS has received grant support from Recor Medical, Ablative Solutions, Applied Therapeutics, Vascular Dynamics, and MSD; and has received consulting fees from Recor Medical, Esperion Inc., Daiichi Sankyo Inc., Novartis, and Vifor Pharma. LT is an employee of NAMSA, a contractor for Recor Medical. MM and LC are employees of Recor Medical. RES has received research grants and personal fees from Recor Medical, Medtronic, and Ablative Solutions.
Figures
Comment in
-
Long-term results of the RADIANCE-HTN TRIO trial: strengths and limitations.Hypertens Res. 2024 Nov;47(11):3277-3279. doi: 10.1038/s41440-024-01903-4. Epub 2024 Sep 19. Hypertens Res. 2024. PMID: 39379468 No abstract available.
References
-
- Kirtane AJ, Sharp ASP, Mahfoud F, Fisher NDL, Schmieder RE, Daemen J, et al. Patient-level pooled analysis of ultrasound renal denervation in the sham-controlled RADIANCE II, RADIANCE-HTN SOLO, and RADIANCE-HTN TRIO Trials. JAMA Cardiol. 2023;8:464–73. 10.1001/jamacardio.2023.0338. - DOI - PMC - PubMed
-
- Azizi M, Sharp ASP, Fisher NDL, Weber MA, Lobo MD, Daemen J, et al. Patient-level pooled analysis of endovascular ultrasound renal denervation or a sham procedure at 6 months following medication escalation: The RADIANCE Clinical Trial Program. Circulation. 2024;149:747–59. 10.1161/CIRCULATIONAHA.123.066941. - DOI - PubMed
-
- Blood Pressure Lowering Treatment Trialists C. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–8. 10.1016/S0140-6736(14)61212-5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical