A human-serum-free medium can induce more infectious P. falciparum gametocytes than a conventional human-serum-containing medium
- PMID: 39333737
- PMCID: PMC11436888
- DOI: 10.1038/s41598-024-73843-5
A human-serum-free medium can induce more infectious P. falciparum gametocytes than a conventional human-serum-containing medium
Abstract
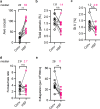
Malaria remains a global health problem, and the standard membrane feeding assay (SMFA) is a key functional assay for development of new interventions to stop malaria transmission from human to mosquito. For SMFA, media with ~ 10% of human serum has been used for infectious gametocyte cultures, however, there are multiple challenges to obtain a suitable human serum. Here we show a human-serum-free culture medium (HSF), which was a mixture of two stem cell culture media and AlbuMAX, supported infectious gametocyte growth. Moreover, the HSF-induced gametocytes elicited significantly higher numbers of oocysts compared to gametocytes cultured with conventional human serum medium (Conv). While some caution is required when comparing percent transmission reducing activity data generated from HSF-SMFA and Conv-SMFA, the HSF method can facilitate the establishment of gametocyte cultures or SMFA by bypassing the need for human serum. Thus, this study will support future development of P. falciparum transmission-blocking interventions.
Keywords: Plasmodium Falciparum: malaria; Gametocyte; Gametocyte culture; SMFA; Serum free; Standard membrane-feeding assay; Transmission-blocking.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. https:World Malaria Report (2023). https://www.who.int/teams/global-malaria-programme/reports/world-malaria... (Accessed on May 17, 2024).
-
- Sinden, R. E. A biologist’s perspective on malaria vaccine development. Hum. Vaccin. 6, 3–11 (2010). - PubMed
-
- van der Boor, S. C. et al. Safety, tolerability, and Plasmodium Falciparum transmission-reducing activity of monoclonal antibody TB31F: a single-centre, open-label, first-in-human, dose-escalation, phase 1 trial in healthy malaria-naive adults. Lancet Infect. Dis. 22, 1596–1605. 10.1016/S1473-3099(22)00428-5 (2022). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

