The TrkC-PTPσ complex governs synapse maturation and anxiogenic avoidance via synaptic protein phosphorylation
- PMID: 39333774
- PMCID: PMC11574141
- DOI: 10.1038/s44318-024-00252-9
The TrkC-PTPσ complex governs synapse maturation and anxiogenic avoidance via synaptic protein phosphorylation
Abstract
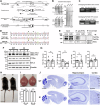
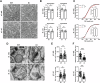
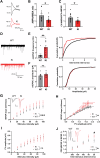
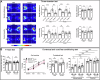
The precise organization of pre- and postsynaptic terminals is crucial for normal synaptic function in the brain. In addition to its canonical role as a neurotrophin-3 receptor tyrosine kinase, postsynaptic TrkC promotes excitatory synapse organization through interaction with presynaptic receptor-type tyrosine phosphatase PTPσ. To isolate the synaptic organizer function of TrkC from its role as a neurotrophin-3 receptor, we generated mice carrying TrkC point mutations that selectively abolish PTPσ binding. The excitatory synapses in mutant mice had abnormal synaptic vesicle clustering and postsynaptic density elongation, more silent synapses, and fewer active synapses, which additionally exhibited enhanced basal transmission with impaired release probability. Alongside these phenotypes, we observed aberrant synaptic protein phosphorylation, but no differences in the neurotrophin signaling pathway. Consistent with reports linking these aberrantly phosphorylated proteins to neuropsychiatric disorders, mutant TrkC knock-in mice displayed impaired social responses and increased avoidance behavior. Thus, through its regulation of synaptic protein phosphorylation, the TrkC-PTPσ complex is crucial for the maturation, but not formation, of excitatory synapses in vivo.
Keywords: Avoidance Behavior; Neurotrophin Receptor; Protein Phosphorylation; Social Novelty; Synapse Organizer.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Alonso P, Gratacos M, Menchon JM, Segalas C, Gonzalez JR, Labad J, Bayes M, Real E, de Cid R, Pertusa A et al (2008) Genetic susceptibility to obsessive-compulsive hoarding: the contribution of neurotrophic tyrosine kinase receptor type 3 gene. Genes Brain Behav 7:778–785 - PubMed
-
- Armengol L, Gratacos M, Pujana MA, Ribases M, Martin-Santos R, Estivill X (2002) 5′ UTR-region SNP in the NTRK3 gene is associated with panic disorder. Mol Psychiatry 7:928–930 - PubMed
MeSH terms
Substances
Grants and funding
- the Research Cluster program/University of Tokushima ()
- Doctoral scholarship/Institut de Recherche Clinique De Montréal (IRCM)
- 252652/FRQ | Fonds de Recherche du Québec - Santé (FRQS)
- JP22H02970/MEXT | Japan Society for the Promotion of Science (JSPS)
- RGPIN-2017-04753/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- Doctoral award/Société Alzheimer | Alzheimer Society Research Program (ASRP)
- MOP-133517/Canadian Government | Canadian Institutes of Health Research (CIHR)
- Junior 2 (29106)/FRQ | Fonds de Recherche du Québec - Santé (FRQS)
- Senior (251655)/FRQ | Fonds de Recherche du Québec - Santé (FRQS)
- R01MH077303/HHS | NIH | National Institute of Mental Health (NIMH)
- JP21K15314/MEXT | Japan Society for the Promotion of Science (JSPS)
- RGPIN-2021-03612/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- Doctoral scholarship (303256)/FRQ | Fonds de Recherche du Québec - Santé (FRQS)
- Doctoral and Emmanuel-Triassi scholarships/Institut de Recherche Clinique De Montréal (IRCM)
- HIRAKU-Global Program/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- PTJ-191947/Canadian Government | CIHR | Institute of Neurosciences, Mental Health and Addiction (INMHA)
- R01 MH077303/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

