Pentagalloylglucose alleviates acetaminophen-induced acute liver injury by modulating inflammation via cGAS-STING pathway
- PMID: 39333876
- PMCID: PMC11428449
- DOI: 10.1186/s10020-024-00924-6
Pentagalloylglucose alleviates acetaminophen-induced acute liver injury by modulating inflammation via cGAS-STING pathway
Abstract
Background: The cGAS-STING pathway is an important component of the innate immune system and plays significant role in acetaminophen-induced liver injury (AILI). Pentagalloylglucose (PGG) is a natural polyphenolic compound with various beneficial effects, including anti-cancer, antioxidant, anti-inflammatory, and liver-protective properties; however, whether it can be used for the treatment of AILI and the specific mechanism remain unclear.
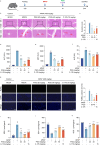
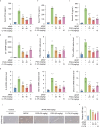
Materials and methods: A cell culture model was created to study the effect of PGG on cGAS-STING pathway activation using various techniques including western blotting (WB), real-time quantitative polymerase chain reaction (RT-qPCR), immunofluorescence (IF), and immunoprecipitation (IP). The effect of PGG was investigated in vivo by establishing a dimethylxanthenone acetic acid (DMXAA)-mediated activation model. An AILI model was used to evaluate the hepatoprotective and therapeutic effects of PGG by detecting liver function indicators, liver histopathology, and cGAS-STING pathway-related indicators in mice with AILI.
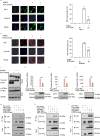
Results: PGG blocked cGAS-STING pathway activation in bone marrow-derived macrophages (BMDMs), THP-1 cells, and peripheral blood mononuclear cells (PBMCs) in vitro. Furthermore, PGG inhibited the generation of type I interferons (IFN-I) and the secretion of inflammatory factors in DMXAA-induced in vivo experiments. In addition, PGG also reduced serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), improved liver tissue damage and apoptosis, and inhibited the cGAS-STING pathway activation caused by acetaminophen. In terms of the mechanism, PGG disrupted the connection between STING and TBK1.
Conclusions: PGG exerts a protective effect against AILI by blocking the cGAS-STING pathway, offering a promising treatment strategy.
Keywords: Acetaminophen; Acute liver injury; Pentagalloylglucose; cGAS-STING pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors have stated that they do not have any conflicts of interest with any organization or individual that could potentially impact the outcomes and tendency of this research.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

