Association between humoral serological markers levels and risk of SARS-CoV-2 infection after the primary COVID-19 vaccine course among ANRS0001S COV-POPART cohort participants
- PMID: 39333909
- PMCID: PMC11429529
- DOI: 10.1186/s12879-024-09861-5
Association between humoral serological markers levels and risk of SARS-CoV-2 infection after the primary COVID-19 vaccine course among ANRS0001S COV-POPART cohort participants
Abstract
Background: We assessed the prognostic value of serological humoral markers measured one month after the last dose of the primary COVID-19 vaccine course for predicting the risk of severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 infection over the following six months in specific populations.
Methods: ANRS0001SCOV-POPART is a French nationwide multicenter prospective observational cohort study assessing the immune response to Covid-19 vaccines routinely administered to 11 subgroups of patients with chronic disease and a control group. Participants from the ANRS0001S COV-POPART were included if they received at least two doses of Covid-19 vaccine for the primary vaccine course, had measurements of anti-Spike, anti-receptor binding domain (RBD) IgG-specific or neutralizing antibodies one month after the end of the primary vaccine course, without being infected by SARS-CoV-2 before the measurement. SARS-CoV-2 infections defined by a positive PCR/antigenic test or seroconversion to detectable anti nucleocapsid antibodies were evaluated until the first COVID-19 booster injection. Cox proportional hazards models taking into account interval-censored data were implemented to estimate the association between each antibody level and the risk of SARS-CoV-2 infection. Predictive performances were evaluated by the area under the receiving operating characteristic curve (AUROC).
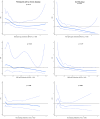
Results: Two thousand five hundred seventy adults from a specific population and 1,123 from the control group were included. The cumulative probabilities of SARS-CoV-2 infections at five months after serological measurement were 6.0% 95% confidence interval: [5.0; 7.9] and 10.1% 95% confidence interval: [8.3; 11.9], respectively. Higher levels of anti-Spike IgG antibody were associated with a lower risk of SARS-CoV-2 infections in the control group, but not in the specific populations. Among the specific populations, AUROC were 74.5%, 74.9%, and 72.4% for anti-Spike IgG, anti-RBD IgG, and neutralizing antibodies, respectively. AUROC were superior in the specific populations, 82.0%, 81.2%, and 81.4% for anti-Spike IgG, anti-RBD IgG, and neutralizing antibodies, respectively.
Conclusions: Vaccine-induced antibody response after the primary course of Covid-19 infection only moderately discriminated between participants developing a SARS-CoV-2 infection during the Omicron wave.
Trial registration: NCT04824651 (first posted: 2021-04-01).
Keywords: Prediction; SARS-CoV-2; Specific populations; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
PL has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Pfizer, Sanofi Pasteur and Support for attending meetings and/or travel from Pfizer, Sanofi Pasteur. JM has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie, Biogen, BMS, Boerhinger Ingelheim, Galapagos, GSK, Fresenius Kabi, Lilly, Mylan, Novartis, Pfizer, Sanofi and grants outside the submitted work from Lilly, Novartis. JPS has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer and Astrazeneca. OL has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi Pasteur; Pfizer, Janssen and, non-financial support from Sanofi Pasteur; Pfizer, Janssen, GlaxoSmithKline and grant from GlaxoSmithKline. The other authors declare having no conflict of interest.
Figures

References
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet Lond Engl. 2021;397(10269):99–111. - DOI - PMC - PubMed
-
- Haute Autorité de Santé. Avis n° 2022.0004/AC/SESPEV du 13 janvier 2022 du collège de la Haute Autorité de santé relatif à la place du vaccin Janssen en seconde dose et en dose de rappel dans la stratégie de vaccination contre la Covid-19. Available from: https://www.has-sante.fr/jcms/p_3309607/fr/avis-n-2022-0004/ac/sespev-du.... Cited 2023 Jan 17.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

