Safety and efficacy of sparsentan versus irbesartan in focal segmental glomerulosclerosis and IgA nephropathy: a systematic review and meta-analysis of randomized controlled trials
- PMID: 39333921
- PMCID: PMC11429118
- DOI: 10.1186/s12882-024-03713-9
Safety and efficacy of sparsentan versus irbesartan in focal segmental glomerulosclerosis and IgA nephropathy: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Sparsentan has shown positive effects on managing different subtypes of glomerulonephritis. The recent results of trials require a pooled analysis to validate these results.
Aim: We aim to assess the safety and efficacy of sparsentan versus irbesartan for patients with IgA nephropathy and focal glomerulosclerosis (FSGS).
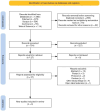
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials retrieved by systematically searching PubMed, Web of Science, Scopus, and Cochrane through March 2024. We used Review Manager v.5.4 to pool dichotomous data using risk ratio (RR) and continuous data using mean difference (MD) with a 95% confidence interval (CI).
Results: Three studies with a total of 884 patients were included. Sparsentan was superior to irbesartan in improving urine protein to creatinine ratio (UP/C) (ratio of percentage reduction 0.66, 95% CI [0.58 to 0.74], P < 0.001); as well as the proportion of patients achieved complete and partial remission of proteinuria (RR = 2.57, 95% CI [1.73 to 3.81], P < 0.001) and (RR = 1.63, 95% CI [1.4 to 1.91], P < 0.001) respectively. Regarding the effect on the glomerular filtration rate, the results estimate did not favor either sparsentan or irbesartan (MD = 1.98 ml/min per 1.73mm2, 95% CI [-1.05 to 5.01], P = 0.2). There were no significant differences in adverse events except for hypotension, which showed higher rates in the sparsentan group (RR = 2.02, 95% CI [1.3 to 3.16], P = 0.002).
Conclusion: Sparsentan is effective and has a good safety profile for treating FSGS and patients with IgA nephropathy. However, more well-designed RCTs against ARBs, ACE inhibitors, and steroids with larger sample sizes are needed to get conclusive evidence.
Keywords: ACE inhibitors; ARBs; Dual endothelin; Focal glomerulosclerosis; IgA nephropathy; Sparsentan.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
The authors declare no competing interests.
Figures
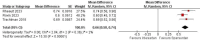

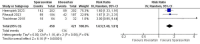
References
-
- Keskinyan VS, Lattanza B, Reid-Adam J. Glomerulonephritis Pediatr Rev. 2023;44:498–512. - PubMed
-
- Wetmore JB, Guo H, Liu J, Collins AJ, Gilbertson DT. The incidence, prevalence, and outcomes of glomerulonephritis derived from a large retrospective analysis. Kidney Int. 2016;90:853–60. - PubMed
-
- Schena FP, Nistor I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin Nephrol. 2018;38:435–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

