Implementing multiple imputations for addressing missing data in multireader multicase design studies
- PMID: 39333923
- PMCID: PMC11428558
- DOI: 10.1186/s12874-024-02321-3
Implementing multiple imputations for addressing missing data in multireader multicase design studies
Abstract
Background: In computer-aided diagnosis (CAD) studies utilizing multireader multicase (MRMC) designs, missing data might occur when there are instances of misinterpretation or oversight by the reader or problems with measurement techniques. Improper handling of these missing data can lead to bias. However, little research has been conducted on addressing the missing data issue within the MRMC framework.
Methods: We introduced a novel approach that integrates multiple imputation with MRMC analysis (MI-MRMC). An elaborate simulation study was conducted to compare the efficacy of our proposed approach with that of the traditional complete case analysis strategy within the MRMC design. Furthermore, we applied these approaches to a real MRMC design CAD study on aneurysm detection via head and neck CT angiograms to further validate their practicality.
Results: Compared with traditional complete case analysis, the simulation study demonstrated the MI-MRMC approach provides an almost unbiased estimate of diagnostic capability, alongside satisfactory performance in terms of statistical power and the type I error rate within the MRMC framework, even in small sample scenarios. In the real CAD study, the proposed MI-MRMC method further demonstrated strong performance in terms of both point estimates and confidence intervals compared with traditional complete case analysis.
Conclusion: Within MRMC design settings, the adoption of an MI-MRMC approach in the face of missing data can facilitate the attainment of unbiased and robust estimates of diagnostic capability.
Keywords: Computer-aided diagnosis; Missing data; Multiple imputation; Multireader multicase.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
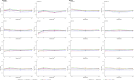
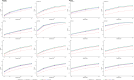
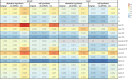
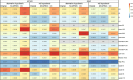

Similar articles
-
Multireader multicase reader studies with binary agreement data: simulation, analysis, validation, and sizing.J Med Imaging (Bellingham). 2014 Oct;1(3):031011. doi: 10.1117/1.JMI.1.3.031011. Epub 2014 Dec 4. J Med Imaging (Bellingham). 2014. PMID: 26158051 Free PMC article.
-
Multireader multicase variance analysis for binary data.J Opt Soc Am A Opt Image Sci Vis. 2007 Dec;24(12):B70-80. doi: 10.1364/josaa.24.000b70. J Opt Soc Am A Opt Image Sci Vis. 2007. PMID: 18059916
-
Reducing the number of reader interpretations in MRMC studies.Acad Radiol. 2009 Feb;16(2):209-17. doi: 10.1016/j.acra.2008.05.014. Acad Radiol. 2009. PMID: 19124107
-
Multireader Diagnostic Accuracy Imaging Studies: Fundamentals of Design and Analysis.Radiology. 2022 Apr;303(1):26-34. doi: 10.1148/radiol.211593. Epub 2022 Feb 15. Radiology. 2022. PMID: 35166584 Review.
-
Review: a gentle introduction to imputation of missing values.J Clin Epidemiol. 2006 Oct;59(10):1087-91. doi: 10.1016/j.jclinepi.2006.01.014. Epub 2006 Jul 11. J Clin Epidemiol. 2006. PMID: 16980149 Review.
References
-
- Wagner RF, Metz CE, Campbell G. Assessment of medical imaging systems and computer aids: a tutorial review. Acad Radiol. 2007;14(6):723–48. - PubMed
-
- Beam CA, Layde PM, Sullivan DC. Variability in the interpretation of screening mammograms by US radiologists. Findings from a national sample. Arch Intern Med. 1996;156(2):209–13. - PubMed
-
- Yu T, Li Q, Gray G, Yue LQ. Statistical innovations in diagnostic device evaluation. J Biopharm Stat. 2016;26(6):1067–77. - PubMed
-
- Clinical Performance Assessment. Considerations for Computer-Assisted Detection Devices Applied to Radiology Images and Radiology Device Data in Premarket Notification (510(k)) Submissions: Guidance for Industry and Food and Drug Administration Staff [https://www.fda.gov/media/77642/download].
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

