Adverse reactions of immune checkpoint inhibitors combined with Proton pump inhibitors: a pharmacovigilance analysis of drug-drug interactions
- PMID: 39334098
- PMCID: PMC11438026
- DOI: 10.1186/s12885-024-12947-7
Adverse reactions of immune checkpoint inhibitors combined with Proton pump inhibitors: a pharmacovigilance analysis of drug-drug interactions
Abstract
Background: Combining immune checkpoint and proton pump inhibitors is widely used in cancer treatment. However, the drug-drug interactions of these substances are currently unknown. This study aimed to explore drug-drug interactions associated with concomitant immune checkpoint and proton pump inhibitors.
Methods: Data were obtained from the US Food and Drug Administration Adverse Event Reporting System from 2014 to 2023. Disproportionality analysis was used for data mining by calculating the reporting odds ratios (RORs) with 95% confidence intervals (95%Cls). The adjusted RORs (RORadj) were then analysed using logistic regression analysis, considering age, sex, and reporting year. Drug-drug interactions occur when a combination treatment enhances the frequency of an event. Further confirmation of the robustness of the findings was achieved using additive and multiplicative models, which are the two statistical methodologies for signal detection of DDIs using spontaneous reporting system.
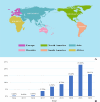
Results: The total number of reports on immune checkpoint combined with proton pump inhibitors was 4,276. Median patient age was 66 years (interquartile range [IQR]: 60-74 years). Significant interaction signals were observed for congenital, familial and genetic disorders (RORadj = 2.66, 95%CI, 1.38-5.14, additive models = 0.7322, multiplicative models = 3.5142), hepatobiliary disorders (RORcrude = 6.64, 95%CI, 5.82-7.58, RORadj = 7.10, 95%CI, 6.16-8.18, additive models = 2.0525, multiplicative models = 1.1622), metabolism and nutrition disorders (RORcrude = 3.27, 95%CI, 2.90-3.69, RORadj = 2.66, 95%CI, 2.30-3.08, additive models = 0.6194), and skin and subcutaneous tissue disorders (RORcrude = 1.41, 95%CI, 1.26-1.58, RORadj = 1.53, 95%CI, 1.34-1.75, additive models = 0.6927, multiplicative models = 5.3599). Subset data analysis showed that programmed death-1 combined with proton pump inhibitors was associated with congenital, familial, and genetic disorders; hepatobiliary disorders; and skin and subcutaneous tissue disorders. Programmed death ligand-1 combined with proton pump inhibitors was associated with adverse reactions of metabolism and nutrition disorders. Cytotoxic T-lymphocyte antigen-4 combined with proton pump inhibitors was associated with congenital, familial, and genetic disorders, and skin and subcutaneous tissue disorders.
Conclusions: Based on real-world data, four Standardized MedDRA Query System Organ Class toxicities were identified as drug-drug interactions associated with combining immune checkpoint and proton pump inhibitors. Clinicians should be cautious when administering these drugs concomitantly. Preclinical trials and robust clinical studies are required to explore the mechanisms and relationships underlying interactions, thus improving understanding of drug-drug interactions associated with this combination therapy.
Keywords: Drug-drug interaction; FDA (Food and Drug Administration) adverse event reporting System (FAERS); Immune checkpoint inhibitors; Pharmacovigilance; Proton pump inhibitors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Michielin O, Lalani AK, Robert C, Sharma P, Peters S. Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. J Immunother Cancer. 2022;10(1):e003024. doi: 10.1136/jitc-2021-003024. Erratum in: J Immunother Cancer. 2022;10(12): PMID: 35078922; PMCID: PMC8796265. - PMC - PubMed
-
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39. 10.1056/NEJMoa1507643. Epub 2015 Sep 27. PMID: 26412456; PMCID: PMC5705936. - PMC - PubMed
-
- Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O’Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus Ipilimumab in Advanced Non-small-cell Lung Cancer. N Engl J Med. 2019;381(21):2020–31. doi: 10.1056/NEJMoa1910231. Epub 2019 Sep 28. PMID: 31562796. - PubMed
-
- Santoni M, Rizzo A, Kucharz J, Mollica V, Rosellini M, Marchetti A, Tassinari E, Monteiro FSM, Soares A, Molina-Cerrillo J, Grande E, Battelli N, Massari F. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–79. 10.1007/s00262-022-03349-4. Epub 2023 Jan 12. PMID: 36633661. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

