Enhancing organoid culture: harnessing the potential of decellularized extracellular matrix hydrogels for mimicking microenvironments
- PMID: 39334251
- PMCID: PMC11429032
- DOI: 10.1186/s12929-024-01086-7
Enhancing organoid culture: harnessing the potential of decellularized extracellular matrix hydrogels for mimicking microenvironments
Abstract
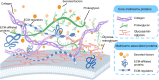
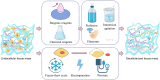
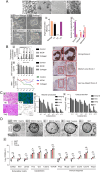
Over the past decade, organoids have emerged as a prevalent and promising research tool, mirroring the physiological architecture of the human body. However, as the field advances, the traditional use of animal or tumor-derived extracellular matrix (ECM) as scaffolds has become increasingly inadequate. This shift has led to a focus on developing synthetic scaffolds, particularly hydrogels, that more accurately mimic three-dimensional (3D) tissue structures and dynamics in vitro. The ECM-cell interaction is crucial for organoid growth, necessitating hydrogels that meet organoid-specific requirements through modifiable physical and compositional properties. Advanced composite hydrogels have been engineered to more effectively replicate in vivo conditions, offering a more accurate representation of human organs compared to traditional matrices. This review explores the evolution and current uses of decellularized ECM scaffolds, emphasizing the application of decellularized ECM hydrogels in organoid culture. It also explores the fabrication of composite hydrogels and the prospects for their future use in organoid systems.
Keywords: Decellularized Extracellular Matrix; Hydrogels; Microenvironment; Natural-based biomaterials; Organoid culture.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Tissue-specific decellularized extracellular matrix rich in collagen, glycoproteins, and proteoglycans and its applications in advanced organoid engineering: A review.Int J Biol Macromol. 2025 Jun;315(Pt 2):144469. doi: 10.1016/j.ijbiomac.2025.144469. Epub 2025 May 22. Int J Biol Macromol. 2025. PMID: 40409619 Review.
-
Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture.Nat Commun. 2019 Dec 11;10(1):5658. doi: 10.1038/s41467-019-13605-4. Nat Commun. 2019. PMID: 31827102 Free PMC article.
-
Natural Hydrogels Support Kidney Organoid Generation and Promote In Vitro Angiogenesis.Adv Mater. 2024 Aug;36(34):e2400306. doi: 10.1002/adma.202400306. Epub 2024 Jun 12. Adv Mater. 2024. PMID: 38762768
-
Binary fabrication of decellularized lung extracellular matrix hybridgels for in vitro chronic obstructive pulmonary disease modeling.Acta Biomater. 2024 Sep 1;185:190-202. doi: 10.1016/j.actbio.2024.07.014. Epub 2024 Jul 24. Acta Biomater. 2024. PMID: 39059731
-
Decellularized Extracellular Matrix for Remodeling Bioengineering Organoid's Microenvironment.Small. 2023 Jun;19(25):e2207752. doi: 10.1002/smll.202207752. Epub 2023 Mar 16. Small. 2023. PMID: 36929582 Review.
Cited by
-
Restoring tendon microenvironment in tendinopathy: Macrophage modulation and tendon regeneration with injectable tendon hydrogel and tendon-derived stem cells exosomes.Bioact Mater. 2025 Jan 22;47:152-169. doi: 10.1016/j.bioactmat.2025.01.016. eCollection 2025 May. Bioact Mater. 2025. PMID: 39906648 Free PMC article.
-
Optimizing protein yield from growing deer antlers.Front Bioeng Biotechnol. 2025 Jun 27;13:1612239. doi: 10.3389/fbioe.2025.1612239. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40657160 Free PMC article.
-
Bottom-up Biomaterial strategies for creating tailored stem cells in regenerative medicine.Front Bioeng Biotechnol. 2025 May 20;13:1581292. doi: 10.3389/fbioe.2025.1581292. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40462840 Free PMC article. Review.
-
Optimal Production of 3D Neuronal Lineage Population by Morphological Classification.Tissue Eng Regen Med. 2025 Jul;22(5):661-674. doi: 10.1007/s13770-025-00721-0. Epub 2025 May 13. Tissue Eng Regen Med. 2025. PMID: 40358835
-
Biomaterial-assisted organoid technology for disease modeling and drug screening.Mater Today Bio. 2024 Dec 31;30:101438. doi: 10.1016/j.mtbio.2024.101438. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39866785 Free PMC article. Review.
References
-
- Kim BS, Das S, Jang J, Cho DW. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem Rev. 2020;120(19):10608–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

