Cytokine priming enhances the antifibrotic effects of human adipose derived mesenchymal stromal cells conditioned medium
- PMID: 39334258
- PMCID: PMC11438190
- DOI: 10.1186/s13287-024-03916-9
Cytokine priming enhances the antifibrotic effects of human adipose derived mesenchymal stromal cells conditioned medium
Abstract
Background: Fibrosis is a pathological scarring process characterized by persistent myofibroblast activation with excessive accumulation of extracellular matrix (ECM). Fibrotic disorders represent an increasing burden of disease-associated morbidity and mortality worldwide for which there are limited therapeutic options. Reversing fibrosis requires the elimination of myofibroblasts, remodeling of the ECM, and regeneration of functional tissue. Multipotent mesenchymal stromal cells (MSC) have antifibrotic properties mediated by secreted factors present in their conditioned medium (MSC-CM). However, there are no standardized in vitro assays to predict the antifibrotic effects of human MSC. As a result, we lack evidence on the effect of cytokine priming on MSC's antifibrotic effects. We hypothesize that the MSC-CM promotes fibrosis resolution in vitro and that this effect is enhanced following MSC cytokine priming.
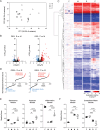
Methods: We compared the antifibrotic effects of resting versus interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) primed MSC-CM in four in vitro assays: prevention of fibroblast activation, myofibroblasts deactivation, ECM degradation and fibrosis resolution in lung explant cultures. Furthermore, we performed transcriptomic analysis of myofibroblasts treated or not with resting or primed MSC-CM and proteomic characterization of resting and primed MSC-CM.
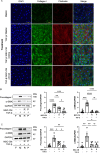
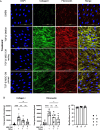
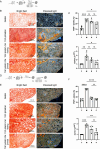
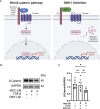
Results: We isolated MSC from adipose tissue of 8 donors, generated MSC-CM and tested each MSC-CM independently. We report that MSC-CM treatment prevented TGF-β induced fibroblast activation to a similar extent as nintedanib but, in contrast to nintedanib, MSC-CM reduced fibrogenic myofibroblasts (i.e. transcriptomic upregulation of apoptosis, senescence, and inflammatory pathways). These effects were larger when primed rather than resting MSC-CM were used. Priming increased the ability of MSC-CM to remodel the ECM, reducing its content of collagen I and fibronectin, and reduced the fibrotic load in TGF-β treated lung explant cultures. Priming increased the following antifibrotic proteins in MSC-CM: DKK1, MMP-1, MMP-3, follistatin and cathepsin S. Inhibition of DKK1 reduced the antifibrotic effects of MSC-CM.
Conclusions: In vitro, MSC-CM promote fibrosis resolution, an effect enhanced following MSC cytokine priming. Specifically, MSC-CM reduces fibrogenic myofibroblasts through apoptosis, senescence, and by enhancing ECM degradation. Future studies will establish the in vivo relevance of MSC priming to fibrosis resolution.
Keywords: Adipose tissue; Conditioned medium; Cytokine priming; Extracellular matrix; Fibroblasts; Fibrosis; Multipotent mesenchymal stromal cells; Myofibroblasts.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

