An oncolytic HSV-1 vector induces a therapeutic adaptive immune response against glioblastoma
- PMID: 39334370
- PMCID: PMC11430576
- DOI: 10.1186/s12967-024-05650-5
An oncolytic HSV-1 vector induces a therapeutic adaptive immune response against glioblastoma
Abstract
Background: Glioblastoma (GBM) is the most frequent and aggressive brain tumor in adults with the lowest survival rates five years post-diagnosis. Oncolytic viruses (OVs) selectively target and damage cancer cells, and for this reason they are being investigated as new therapeutic tools also against GBM.
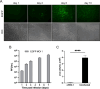
Methods: An oncolytic herpes simplex virus type 1 (oHSV-1) with deletions in the γ34.5 neurovirulence gene and the US12 gene, expressing enhanced green fluorescent protein (EGFP-oHSV-1) as reporter gene was generated and tested for its capacity to infect and kill the murine GL261 glioblastoma (GBM) cell line. Syngeneic mice were orthotopically injected with GL261cells. Seven days post-implantation, EGFP-oHSV-1 was administered intratumorally. Twenty-one days after parental tumor challenge in the opposite brain hemisphere, mice were sacrified and their brains were analysed by immunohistochemistry to assess tumor presence and cell infiltrate.
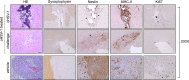
Results: oHSV-1 replicates and induces cell death of GL261 cells in vitro. A single intracranial injection of EGFP-oHSV-1 in established GL261 tumors significantly prolongs survival in all treated mice compared to placebo treatment. Notably, 45% of treated mice became long-term survivors, and rejected GL261 cells upon rechallenge in the contralateral brain hemisphere, indicating an anamnestic antitumoral immune response. Post-mortem analysis revealed a profound modification of the tumor microenvironment with increased infiltration of CD4 + and CD8 + T lymphocytes, intertumoral vascular collapse and activation and redistribution of macrophage, microglia, and astroglia in the tumor area, with the formation of intense fibrotic tissue suggestive of complete rejection in long-term survivor mice.
Conclusions: EGFP-oHSV1 demonstrates potent antitumoral activity in an immunocompetent GBM model as a monotherapy, resulting from direct cell killing combined with the stimulation of a protective adaptive immune response. These results open the way to possible application of our strategy in clinical setting.
Keywords: GL261; Glioblastoma; HSV-1; Immunotherapy; Oncolytic virus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

