Human uncultured adipose-derived stromal vascular fraction shows therapeutic potential against osteoarthritis in immunodeficient rats via direct effects of transplanted M2 macrophages
- PMID: 39334434
- PMCID: PMC11438128
- DOI: 10.1186/s13287-024-03946-3
Human uncultured adipose-derived stromal vascular fraction shows therapeutic potential against osteoarthritis in immunodeficient rats via direct effects of transplanted M2 macrophages
Abstract
Background: The uncultured adipose-derived stromal vascular fraction (SVF), consisting of adipose-derived stromal cells (ADSCs), M2 macrophages (M2Φ) and others, has shown therapeutic potential against osteoarthritis (OA), however, the mechanisms underlying its therapeutic effects remain unclear. Therefore, this study investigated the effects of the SVF on OA in a human-immunodeficient rat xenotransplantation model.
Methods: OA model was induced in the knees of female immunodeficient rats by destabilization of the medial meniscus. Immediately after the surgery, human SVF (1 × 105), ADSCs (1 × 104), or phosphate buffered saline as a control group were transplanted into the knees. At 4 and 8 weeks postoperatively, OA progression and synovitis were analyzed by macroscopic and histological analyses, and the expression of collagen II, SOX9, MMP-13, ADAMTS-5, F4/80, CD86 (M1), CD163 (M2), and human nuclear antigen (hNA) were evaluated immunohistochemically. In vitro, flow cytometry was performed to collect CD163-positive cells as M2Φ from the SVF. Chondrocyte pellets (1 × 105) were co-cultured with SVF (1 × 105), M2Φ (1 × 104), and ADSCs (1 × 104) or alone as a control group, and the pellet size was compared. TGF-β, IL-10 and MMP-13 concentrations in the medium were evaluated using enzyme-linked immunosorbent assay.
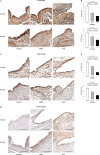
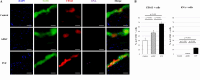
Results: In comparison with the control and ADSC groups, the SVF group showed significantly slower OA progression and less synovitis with higher expression of collagen II and SOX9, lower expression of MMP-13 and ADAMTS-5, and lower F4/80 and M1/M2 ratio in the synovium. Only the SVF group showed partial expression of hNA-, CD163-, and F4/80-positive cells in the rat synovium. In vitro, the SVF, M2Φ, ADSC and control groups, in that order, showed larger pellet sizes, higher TGF-β and IL-10, and lower MMP-13 concentrations.
Conclusions: The M2Φ in the transplanted SVF directly affected recipient tissue, enhancing the secretion of growth factors and chondrocyte-protecting cytokines, and partially improving chondrocytes and joint homeostasis. These findings indicate that the SVF is as an effective option for regenerative therapy for OA, with mechanisms different from those of ADSCs.
Keywords: Adipose-derived stromal cells; M2 macrophages; Osteoarthritis; Rat model; Stromal vascular fraction; Synovitis; Xenotransplantation.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures




References
-
- Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci. 2021;22(8):3851. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

