Development and characterization of reverse genetics systems of feline infectious peritonitis virus for antiviral research
- PMID: 39334482
- PMCID: PMC11438400
- DOI: 10.1186/s13567-024-01373-z
Development and characterization of reverse genetics systems of feline infectious peritonitis virus for antiviral research
Erratum in
-
Correction: Development and characterization of reverse genetics systems of feline infectious peritonitis virus for antiviral research.Vet Res. 2025 Mar 24;56(1):66. doi: 10.1186/s13567-025-01498-9. Vet Res. 2025. PMID: 40128828 Free PMC article. No abstract available.
Abstract
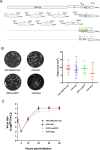
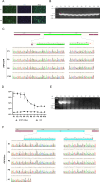
Feline infectious peritonitis (FIP) is a lethal, immune-mediated disease in cats caused by feline infectious peritonitis virus (FIPV), a biotype of feline coronavirus (FCoV). In contrast to feline enteric coronavirus (FECV), which exclusively infects enterocytes and causes diarrhea, FIPV specifically targets macrophages, resulting in the development of FIP. The transmission and infection mechanisms of this complex, invariably fatal disease remain unclear, with no effective vaccines or approved drugs for its prevention or control. In this study, a full-length infectious cDNA clone of the wild-type FIPV WSU79-1149 strain was constructed to generate recombinant FIPV (rFIPV-WT), which exhibited similar growth kinetics and produced infectious virus titres comparable to those of the parental wild-type virus. In addition, the superfold green fluorescent protein (msfGFP) and Renilla luciferase (Rluc) reporter genes were incorporated into the rFIPV-WT cDNA construct to generate reporter rFIPV-msfGFP and rFIPV-Rluc viruses. While the growth characteristics of the rFIPV-msfGFP virus were similar to those of its parental rFIPV-WT, the rFIPV-Rluc virus replicated more slowly, resulting in the formation of smaller plaques than did the rFIPV-WT and rFIPV-msfGFP viruses. In addition, by replacing the S, E, M, and ORF3abc genes with msfGFP and Rluc genes, the replicon systems repFIPV-msfGFP and repFIPV-Rluc were generated on the basis of the cDNA construct of rFIPV-WT. Last, the use of reporter recombinant viruses and replicons in antiviral screening assays demonstrated their high sensitivity for quantifying the antiviral effectiveness of the tested compounds. This integrated system promises to significantly streamline the investigation of virus replication within host cells, enabling efficient screening for anti-FIPV compounds and evaluating emerging drug-resistant mutations within the FIPV genome.
Keywords: Feline infectious peritonitis virus; antiviral compounds; high-content screening; recombinant viruses; replicon; reverse genetics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Woo PCY, de Groot RJ, Haagmans B, Lau SKP, Neuman BW, Perlman S, Sola I, van der Hoek L, Wong ACP, Yeh SH (2023) ICTV virus taxonomy profile: coronaviridae 2023. J Gen Virol 104:001843 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

