Mitigating Doxorubicin-Induced Cardiotoxicity through Quercetin Intervention: An Experimental Study in Rats
- PMID: 39334727
- PMCID: PMC11429272
- DOI: 10.3390/antiox13091068
Mitigating Doxorubicin-Induced Cardiotoxicity through Quercetin Intervention: An Experimental Study in Rats
Abstract
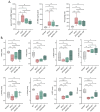
Doxorubicin (DOX) is an effective anticancer drug, but its use is limited by dose-dependent heart toxicity. Quercetin is a natural antioxidant frequently studied for its beneficial properties. Moreover, a wide range of dietary supplements are available for human use. This in vivo study aimed to explore the potential cardioprotective effects of quercetin in chronic DOX treatment. A total of 32 Wistar rats were randomly divided into four groups: control, DOX, DOX/Q-50, and DOX/Q-100, treated with saline, 2.5 mg/kg body-weight DOX, 2.5 mg/kg body-weight DOX + 50 mg quercetin, and 2.5 mg/kg body-weight DOX + 100 mg quercetin, respectively, for two weeks. Rats were monitored using cardiac ultrasound (US) and markers for cardiac injury. Oxidative damage and ultrastructural changes in the heart were investigated. Chronic DOX treatment led to a decline in cardiac function and elevated values of NT pro-BNP, troponin I, and CK-MB. Quercetin treatment slightly improved certain US parameters, and normalized serum NT pro-BNP levels. Furthermore, DOX-induced SOD1 depletion with consequent Nrf2 activation and DNA damage as shown by an increase in γH2AX and 8HOdG. Quercetin treatment alleviated these alterations. Oral administration of quercetin alleviated serum markers associated with DOX-induced cardiotoxicity. Furthermore, it exhibited a favorable impact on the cardiac US parameters. This suggests that quercetin may have potential cardioprotective properties.
Keywords: DNA damage; antineoplastic agents; antioxidants; cardiotoxicity; doxorubicin; microscopy; oxidative stress; quercetin; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
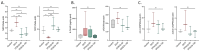


References
-
- Lyon A.R., López-Fernández T., Couch L.S., Asteggiano R., Aznar M.C., Bergler-Klein J., Boriani G., Cardinale D., Cordoba R., Cosyns B., et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the Task Force on Cardio-Oncology of the European Society of Cardiology (ESC) Eur. Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. - DOI - PubMed
-
- Cadeddu C., Mercurio V., Spallarossa P., Nodari S., Triggiani M., Monte I., Piras R., Madonna R., Pagliaro P., Tocchetti C.G., et al. Preventing Antiblastic Drug-Related Cardiomyopathy: Old and New Therapeutic Strategies. J. Cardiovasc. Med. 2016;17:e64. doi: 10.2459/JCM.0000000000000382. - DOI - PubMed
-
- Tan C., Tasaka H., Yu K.-P., Murphy M.L., Karnofsky D.A. Daunomycin, an Antitumor Antibiotic, in the Treatment of Neoplastic Disease. Clinical Evaluation with Special Reference to Childhood Leukemia. Cancer. 1967;20:333–353. doi: 10.1002/1097-0142(1967)20:3<333::AID-CNCR2820200302>3.0.CO;2-K. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

