Beneficial Effect of Dimethyl Fumarate Drug Repositioning in a Mouse Model of TDP-43-Dependent Frontotemporal Dementia
- PMID: 39334731
- PMCID: PMC11428793
- DOI: 10.3390/antiox13091072
Beneficial Effect of Dimethyl Fumarate Drug Repositioning in a Mouse Model of TDP-43-Dependent Frontotemporal Dementia
Abstract
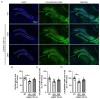
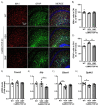
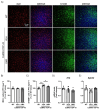
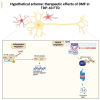
Frontotemporal dementia (FTD) causes progressive neurodegeneration in the frontal and temporal lobes, leading to behavioral, cognitive, and language impairments. With no effective treatment available, exploring new therapeutic approaches is critical. Recent research highlights the transcription factor Nuclear Factor erythroid-derived 2-like 2 (NRF2) as vital in limiting neurodegeneration, with its activation shown to mitigate FTD-related processes like inflammation. Dimethyl fumarate (DMF), an NRF2 activator, has demonstrated neuroprotective effects in a TAU-dependent FTD mouse model, reducing neurodegeneration and inflammation. This suggests DMF repositioning potential for FTD treatment. Until now, no trial had been conducted to analyze the effect of DMF on TDP-43-dependent FTD. In this study, we aimed to determine the potential therapeutic efficacy of DMF in a TDP-43-related FTD mouse model that exhibits early cognitive impairment. Mice received oral DMF treatment every other day from presymptomatic to symptomatic stages. By post-natal day (PND) 60, an improvement in cognitive function is already evident, becoming even more pronounced by PND90. This cognitive enhancement correlates with the neuroprotection observed in the dentate gyrus and a reduction in astrogliosis in the stratum lacunosum-moleculare zone. At the prefrontal cortex (PFC) level, a neuroprotective effect of DMF is also observed, accompanied by a reduction in astrogliosis. Collectively, our results suggest a potential therapeutic application of DMF for patients with TDP-43-dependent FTD.
Keywords: DMF; NRF2; TDP-43; neurodegeneration; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Leroy M., Bertoux M., Skrobala E., Mode E., Adnet-Bonte C., Le Ber I., Bombois S., Cassagnaud P., Chen Y., Deramecourt V., et al. Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimer’s Res. Ther. 2021;13:19. doi: 10.1186/s13195-020-00753-9. - DOI - PMC - PubMed
-
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

