Challenging Sarcopenia: Exploring AdipoRon in Aging Skeletal Muscle as a Healthspan-Extending Shield
- PMID: 39334732
- PMCID: PMC11428238
- DOI: 10.3390/antiox13091073
Challenging Sarcopenia: Exploring AdipoRon in Aging Skeletal Muscle as a Healthspan-Extending Shield
Abstract
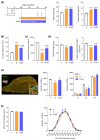
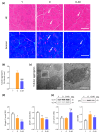
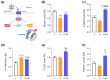
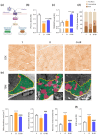
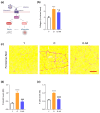
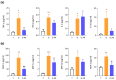
Sarcopenia, characterized by loss of muscle mass, quality, and function, poses significant risks in aging. We previously demonstrated that long-term treatment with AdipoRon (AR), an adiponectin receptor agonist, alleviated myosteatosis and muscle degeneration in middle-aged obese mice. This study aimed to determine if a shorter AR treatment could effectively offset sarcopenia in older mice. Two groups of old mice (20-23 months) were studied, one untreated (O) and one orally-treated with AR (O-AR) at 50 mg/kg/day for three months, compared with control 3-month-old young mice (Y) or 10-month-old young-adult mice (C-10). Results showed that AR remarkably inversed the loss of muscle mass by restoring the sarcopenia index and fiber count, which were greatly diminished with age. Additionally, AR successfully saved muscle quality of O mice by halving the accumulation of tubular aggregates and aberrant mitochondria, through AMPK pathway activation and enhanced autophagy. AR also bolstered muscle function by rescuing mitochondrial activity and improving exercise endurance. Finally, AR markedly curbed muscle fibrosis and mitigated local/systemic inflammation. Thus, a late three-month AR treatment successfully opposed sarcopenia and counteracted various hallmarks of aging, suggesting AR as a promising anti-aging therapy for skeletal muscles, potentially extending healthspan.
Keywords: AMPK; abnormal mitochondria; adiponectin; adiponectin agonist; autophagy; sarcopenia; tubular aggregates.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Otabe S., Yuan X., Fukutani T., Wada N., Hashinaga T., Nakayama H., Hirota N., Kojima M., Yamada K. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am. J. Physiology. Endocrinol. Metab. 2007;293:E210–E218. doi: 10.1152/ajpendo.00645.2006. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

