Unraveling the Protective Role of Oleocanthal and Its Oxidation Product, Oleocanthalic Acid, against Neuroinflammation
- PMID: 39334733
- PMCID: PMC11428454
- DOI: 10.3390/antiox13091074
Unraveling the Protective Role of Oleocanthal and Its Oxidation Product, Oleocanthalic Acid, against Neuroinflammation
Abstract
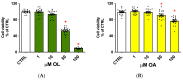
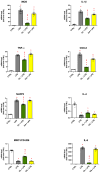
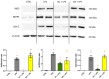
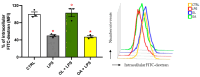
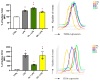
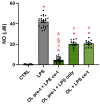
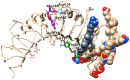
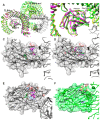
Neuroinflammation is a critical aspect of various neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases. This study investigates the anti-neuroinflammatory properties of oleocanthal and its oxidation product, oleocanthalic acid, using the BV-2 cell line activated with lipopolysaccharide. Our findings revealed that oleocanthal significantly inhibited the production of pro-inflammatory cytokines and reduced the expression of inflammatory genes, counteracted oxidative stress induced by lipopolysaccharide, and increased cell phagocytic activity. Conversely, oleocanthalic acid was not able to counteract lipopolysaccharide-induced activation. The docking analysis revealed a plausible interaction of oleocanthal, with both CD14 and MD-2 leading to a potential interference with TLR4 signaling. Since our data show that oleocanthal only partially reduces the lipopolysaccharide-induced activation of NF-kB, its action as a TLR4 antagonist alone cannot explain its remarkable effect against neuroinflammation. Proteomic analysis revealed that oleocanthal counteracts the LPS modulation of 31 proteins, including significant targets such as gelsolin, clathrin, ACOD1, and four different isoforms of 14-3-3 protein, indicating new potential molecular targets of the compound. In conclusion, oleocanthal, but not oleocanthalic acid, mitigates neuroinflammation through multiple mechanisms, highlighting a pleiotropic action that is particularly important in the context of neurodegeneration.
Keywords: 14-3-3 protein family; ACOD1; BV-2 microglial cells; TLR4; clathrin; gelsolin; lipopolysaccharide; neuroinflammation; oleocanthal; oleocanthalic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Grants and funding
- PRIN2022 number 20222W7P7S/Ministry of University and Research (MUR)
- PRIN2022 number 2022LW54KC/Ministry of University and Research (MUR)
- PON 2014-2020 "Research and Innovation" resources-Green/Innovation Action-DM MUR 1062/2021-Title of research "Sviluppo di una piattaforma tecnologica per lo studio delle pro-prietà nutraceutiche di bio-molecole e biomateriali presenti negli scarti derivan/Ministry of University and Research (MUR)
LinkOut - more resources
Full Text Sources
Research Materials

