Synthesis, Characterization and Assessment of Antioxidant and Melanogenic Inhibitory Properties of Edaravone Derivatives
- PMID: 39334807
- PMCID: PMC11429142
- DOI: 10.3390/antiox13091148
Synthesis, Characterization and Assessment of Antioxidant and Melanogenic Inhibitory Properties of Edaravone Derivatives
Abstract
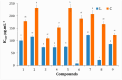
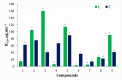
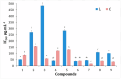
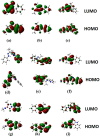
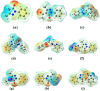
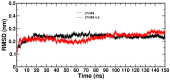
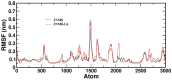
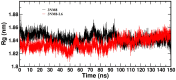
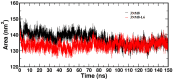
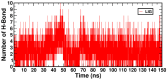
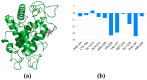
A series of edaravone derivatives and the corresponding Cu(II) complexes were synthesized and characterized using spectroscopic and analytical techniques such as IR, UV, NMR and elemental analysis. Antioxidant activities of all compounds were examined using free radical scavenging methods such as hydrogen peroxide scavenging activity (HPSA), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2-2'-azino-bis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) assays. All of the tested compounds exhibited good antioxidant activity. Further, the frontier orbital energy levels, as well as various chemical properties, were determined using the density functional theory (DFT) calculations. The MEP maps of all of the derivatives were plotted to identify the nucleophilic and electrophilic reactive sites. Further, binding energies of all of the organic compounds with the protein tyrosinase was investigated to determine their potential anti-melanogenic applications. The selected ligand, L6 was subjected to molecular dynamics simulation analysis to determine the stability of the ligand-protein complex. The MD simulation was performed (150 ns) to estimate the stability of the tyrosinase-L6 complex. Other key parameters, such as, RMSD, RMSF, Rg, hydrogen bonds, SASA and MMPBSA were also analyzed to understand the interaction of L6 with the tyrosinase protein.
Keywords: antioxidant activity; copper(II) complexes; edaravone derivatives; melanogenic inhibition activity; molecular docking; molecular dynamics simulation; tyrosinase-binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Canadian Agency for Drugs and Technologies in Health . Pharmacoeconomic Review Report: Edaravone (Radicava): (Mitsubishi Tanabe Pharma Corporation): Indication: For the treatment of Amyotrophic Lateral Sclerosis (ALS) [Internet] Canadian Agency for Drugs and Technologies in Health; Ottawa, ON, Canada: 2019. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

