From Pathogenesis to Treatment: Targeting Type-2 Inflammation in Eosinophilic Esophagitis
- PMID: 39334846
- PMCID: PMC11429508
- DOI: 10.3390/biom14091080
From Pathogenesis to Treatment: Targeting Type-2 Inflammation in Eosinophilic Esophagitis
Abstract
Eosinophilic esophagitis (EoE) is a chronic inflammatory disorder of the esophagus. EoE shares a common pathogenetic mechanism with other chronic disorders pertaining to the type 2 inflammatory spectrum, such as atopic dermatitis (AD), allergic rhinitis (AR), asthma, and chronic rhinosinusitis with nasal polyps (CRSwNP). The recent advancements in EoE pathogenesis understanding have unveiled new molecular targets implied within the "atopic march" picture as well as specific to EoE. These discoveries have led to the clinical evaluation of several novel drugs (monoclonal antibodies and immune modulators), specifically aimed at the modulation of Th2 inflammation. In this comprehensive review, we have focused on the subtle mechanisms of type 2 inflammatory disorders, highlighting the similarities and differences with EoE, taking a deeper look into the evolving field of biologic therapies, already approved or under current investigation.
Keywords: EoE; atopic march; atopy; biologics; eosinophilic esophagitis; type 2 inflammation.
Conflict of interest statement
A.B., F.V.M., M.-R.Y., L.A., L.M., F.U., S.P., and G.M.C.M. have no conflicts of interest to declare related to this article. E.V.S. served as a consultant for Abbvie, Agave, Alfasigma, Biogen, Bristol Myers Squibb, Celltrion, Diadema Farmaceutici, Falk, Fenix Pharma, Fresenius Kabi, Janssen, J.B. Pharmaceuticals, Merck & Co, Nestlè, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Synformulas GmbH, Takeda, and Unifarco; he received research support from Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, and Zeta Farmaceutici. S.D. received consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor. A.J.B. received research funding from Nutricia, Norgine, Thelial, SST, and Falk Pharma and received speaker and/or consulting fees from Laborie, Thelial, EsoCap, Medtronic, Falk Pharma, Calypso Biotech, Alimentiv, Sanofi/Regeneron, Reckett, and AstraZeneca, and E.V. received speaker and/or consulting fees from Sanofi/Regeneron, Falk Pharma, Apollo Therapeutics, and Aurora Biofarma.
Figures

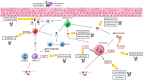
References
-
- Dellon E.S., Gonsalves N., Hirano I., Furuta G.T., A Liacouras C., A Katzka D. ACG Clinical Guideline: Evidenced Based Approach to the Diagnosis and Management of Esophageal Eosinophilia and Eosinophilic Esophagitis (EoE) Am. J. Gastroenterol. 2013;108:679–692. doi: 10.1038/ajg.2013.71. - DOI - PubMed
-
- Hahn J.W., Lee K., Shin J.I., Cho S.H., Turner S., Shin J.U., Yeniova A., Koyanagi A., Jacob L., Smith L., et al. Global Incidence and Prevalence of Eosinophilic Esophagitis, 1976–2022: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023;21:3270–3284.e77. doi: 10.1016/j.cgh.2023.06.005. - DOI - PubMed
-
- Alexander E.S., Martin L.J., Collins M.H., Kottyan L.C., Sucharew H., He H., Mukkada V.A., Succop P.A., Abonia J.P., Foote H., et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2014;134:1084–1092.e1. doi: 10.1016/j.jaci.2014.07.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

