The Role of Claudins in the Pathogenesis of Dextran Sulfate Sodium-Induced Experimental Colitis: The Effects of Nobiletin
- PMID: 39334888
- PMCID: PMC11430412
- DOI: 10.3390/biom14091122
The Role of Claudins in the Pathogenesis of Dextran Sulfate Sodium-Induced Experimental Colitis: The Effects of Nobiletin
Abstract
Background: The pathogenesis of inflammatory bowel diseases such as ulcerative colitis and Crohn's disease is not well understood. This study investigated the roles and regulation of the claudin-1, -2, -3, and -4 isoforms in the pathogenesis of ulcerative colitis, and the potential therapeutic effects of nobiletin.
Methods: Colitis was induced in rats by administering dextran sulfate sodium [DSS] in drinking water for seven days. Animals were treated daily with nobiletin [oral, 60 mg/Kg body weight] and studied in four groups, C [non-colitis control], D [DSS-induced colitis], CN [nobiletin-treated non-colitis control], and DN [nobiletin-treated DSS-induced colitis]. On day seven, the animals were sacrificed, and colonic tissues were collected and analyzed.
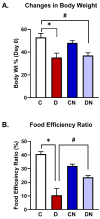
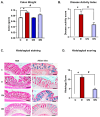
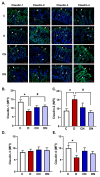
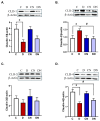
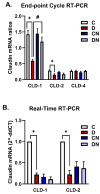
Results: Both macroscopic and microscopic findings suggest the progression of colitis. In the inflamed colon, claudin-1 and -4 proteins were decreased, claudin-2 increased, while the claudin-3 protein remained unchanged. Except for claudin-1, these changes were not paralleled by mRNA expression, indicating a complex regulatory mechanism. Uniform β-actin expression along with consistent quality and yield of total RNA indicated selectivity of these changes. Nobiletin treatment reversed these changes.
Conclusions: Altered expression of the claudin isoforms -1, -2, and -4 disrupts tight junctions, exposing the lamina propria to microflora, leading to electrolyte disturbance and the development of ulcerative colitis. Nobiletin with its anti-inflammatory properties may be useful in IBD.
Keywords: claudins; colitis; dextran sulfate sodium; inflammation; myeloperoxidase; tight junctions.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

