Urinary Extracellular Vesicles for Non-Invasive Quantification of Principal Cell Damage in Kidney Transplant Recipients
- PMID: 39334890
- PMCID: PMC11430813
- DOI: 10.3390/biom14091124
Urinary Extracellular Vesicles for Non-Invasive Quantification of Principal Cell Damage in Kidney Transplant Recipients
Abstract
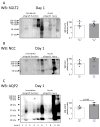
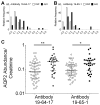
The objective of the present study was to compare principal cell-specific aquaporin-2 (AQP2) abundances in urinary extracellular vesicles (uEVs) on the first postoperative day in deceased-donor kidney transplant recipients without and with acute kidney injury. We measured uEV markers (CD9 and CD63) and the abundances of proximal tubular sodium-glucose transporter 2, distal tubular sodium/chloride cotransporter, and principal cell-specific aquaporin-2 using Western blotting of urine. uEV-AQP2 levels were normalized to living donor controls. The validation cohort consisted of 82 deceased-donor kidney transplant recipients who had a median age of 50 years (IQR 43 to 57 years). A total of 32% of recipients had acute kidney injury. The median uEV-AQP2 was significantly higher in recipients with acute kidney injury compared to immediate allograft function (2.05; IQR 0.87 to 2.83; vs. 0.81; IQR 0.44 to 1.78; p < 0.01). The Youden index indicated a uEV-AQP2 threshold of 2.00. Stratifying uEV-AQP2 into quartiles showed that recipients with higher uEV-AQP2 levels had higher rates of acute kidney injury (Cochran-Armitage, p = 0.001). The discovery cohort showed elevated CD9, CD63, and uEV-AQP2 levels in urine from recipients with acute kidney injury compared to immediate allograft function. We were able to quantify the damage of principal cells after kidney transplant to predict acute kidney injury using uEV-AQP2.
Keywords: allograft injury; aquaporin-2 abundance; damage of principal cells; exosomes; extracellular vesicles; kidney transplant; urinary extracellular vesicle.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Johnston O., O’Kelly P., Spencer S., Donohoe J., Walshe J.J., Little D.M., Hickey D., Conlon P.J. Reduced graft function (with or without dialysis) vs immediate graft function--A comparison of long-term renal allograft survival. Nephrol. Dial. Transplant. 2006;21:2270–2274. doi: 10.1093/ndt/gfl103. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

