Current Treatment Methods for Charcot-Marie-Tooth Diseases
- PMID: 39334903
- PMCID: PMC11430469
- DOI: 10.3390/biom14091138
Current Treatment Methods for Charcot-Marie-Tooth Diseases
Abstract
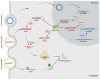
Charcot-Marie-Tooth (CMT) disease, the most common inherited neuromuscular disorder, exhibits a wide phenotypic range, genetic heterogeneity, and a variable disease course. The diverse molecular genetic mechanisms of CMT were discovered over the past three decades with the development of molecular biology and gene sequencing technologies. These methods have brought new options for CMT reclassification and led to an exciting era of treatment target discovery for this incurable disease. Currently, there are no approved disease management methods that can fully cure patients with CMT, and rehabilitation, orthotics, and surgery are the only available treatments to ameliorate symptoms. Considerable research attention has been given to disease-modifying therapies, including gene silencing, gene addition, and gene editing, but most treatments that reach clinical trials are drug treatments, while currently, only gene therapies for CMT2S have reached the clinical trial stage. In this review, we highlight the pathogenic mechanisms and therapeutic investigations of different subtypes of CMT, and promising therapeutic approaches are also discussed.
Keywords: Charcot–Marie–Tooth; drug therapy; gene therapy; pathomechanism; rehabilitation; surgery.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the authorship of this article.
Figures


References
Publication types
MeSH terms
Grants and funding
- 2022YFS0161/Sichuan Provincial Natural Science Foundation of China
- 2023YFS0147/Sichuan Provincial Natural Science Foundation of China
- XZ202201YD0018C/the Central Guiding Local Science and Technology Development Fund project in 2022
- 22HXFH015/1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University
- 82302544/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

