Proteomic Profile of Circulating Extracellular Vesicles in the Brain after Δ9-Tetrahydrocannabinol Inhalation
- PMID: 39334909
- PMCID: PMC11430348
- DOI: 10.3390/biom14091143
Proteomic Profile of Circulating Extracellular Vesicles in the Brain after Δ9-Tetrahydrocannabinol Inhalation
Abstract
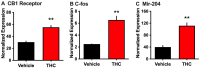
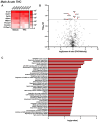
Given the increasing use of cannabis in the US, there is an urgent need to better understand the drug's effects on central signaling mechanisms. Extracellular vesicles (EVs) have been identified as intercellular signaling mediators that contain a variety of cargo, including proteins. Here, we examined whether the main psychoactive component in cannabis, Δ9-tetrahydrocannabinol (THC), alters EV protein signaling dynamics in the brain. We first conducted in vitro studies, which found that THC activates signaling in choroid plexus epithelial cells, resulting in transcriptional upregulation of the cannabinoid 1 receptor and immediate early gene c-fos, in addition to the release of EVs containing RNA cargo. Next, male and female rats were examined for the effects of either acute or chronic exposure to aerosolized ('vaped') THC on circulating brain EVs. Cerebrospinal fluid was extracted from the brain, and EVs were isolated and processed with label-free quantitative proteomic analyses via high-resolution tandem mass spectrometry. Interestingly, circulating EV-localized proteins were differentially expressed based on acute or chronic THC exposure in a sex-specific manner. Taken together, these findings reveal that THC acts in the brain to modulate circulating EV signaling, thereby providing a novel understanding of how exogenous factors can regulate intercellular communication in the brain.
Keywords: THC; cannabis; cerebrospinal fluid; extracellular vesicles; proteomic.
Conflict of interest statement
C.D.F. serves on the scientific advisory board of a therapeutic development company, GATC Health, which is unrelated to the data presented herein. All other authors have no potential conflicts of interest to disclose.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

