Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease
- PMID: 39334952
- PMCID: PMC11430160
- DOI: 10.3390/biom14091186
Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease
Abstract
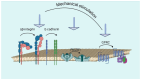
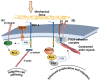
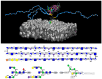
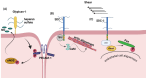
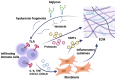
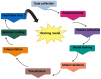
Glycosaminoglycans (GAGs) and proteoglycans (PGs) are essential components of the extracellular matrix (ECM) with pivotal roles in cellular mechanosensing pathways. GAGs, such as heparan sulfate (HS) and chondroitin sulfate (CS), interact with various cell surface receptors, including integrins and receptor tyrosine kinases, to modulate cellular responses to mechanical stimuli. PGs, comprising a core protein with covalently attached GAG chains, serve as dynamic regulators of tissue mechanics and cell behavior, thereby playing a crucial role in maintaining tissue homeostasis. Dysregulation of GAG/PG-mediated mechanosensing pathways is implicated in numerous pathological conditions, including cancer and inflammation. Understanding the intricate mechanisms by which GAGs and PGs modulate cellular responses to mechanical forces holds promise for developing novel therapeutic strategies targeting mechanotransduction pathways in disease. This comprehensive overview underscores the importance of GAGs and PGs as key mediators of mechanosensing in maintaining tissue homeostasis and their potential as therapeutic targets for mitigating mechano-driven pathologies, focusing on cancer and inflammation.
Keywords: cancer; glycosaminoglycans; glypican; inflammation; mechanosensing; mechanotransduction; proteoglycans; syndecans.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Glycosaminoglycans, Instructive Biomolecules That Regulate Cellular Activity and Synaptic Neuronal Control of Specific Tissue Functional Properties.Int J Mol Sci. 2025 Mar 12;26(6):2554. doi: 10.3390/ijms26062554. Int J Mol Sci. 2025. PMID: 40141196 Free PMC article. Review.
-
Matrix glycosaminoglycans in the growth phase of fibroblasts: more of the story in wound healing.J Surg Res. 2000 Jul;92(1):45-52. doi: 10.1006/jsre.2000.5840. J Surg Res. 2000. PMID: 10864481
-
A biological guide to glycosaminoglycans: current perspectives and pending questions.FEBS J. 2024 Aug;291(15):3331-3366. doi: 10.1111/febs.17107. Epub 2024 Mar 18. FEBS J. 2024. PMID: 38500384 Review.
-
A role for proteoglycans in vascular disease.Matrix Biol. 2018 Oct;71-72:396-420. doi: 10.1016/j.matbio.2018.02.019. Epub 2018 Feb 27. Matrix Biol. 2018. PMID: 29499356 Free PMC article. Review.
-
Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression.Int J Mol Sci. 2020 Aug 20;21(17):5983. doi: 10.3390/ijms21175983. Int J Mol Sci. 2020. PMID: 32825245 Free PMC article. Review.
Cited by
-
Decellularized Green and Brown Macroalgae as Cellulose Matrices for Tissue Engineering.J Funct Biomater. 2024 Dec 23;15(12):390. doi: 10.3390/jfb15120390. J Funct Biomater. 2024. PMID: 39728190 Free PMC article.
-
Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes.Gels. 2025 Apr 9;11(4):281. doi: 10.3390/gels11040281. Gels. 2025. PMID: 40277717 Free PMC article. Review.
-
Leveraging Microneedles for Raised Scar Management.Polymers (Basel). 2025 Jan 2;17(1):108. doi: 10.3390/polym17010108. Polymers (Basel). 2025. PMID: 39795511 Free PMC article. Review.
-
Glycosaminoglycans, Instructive Biomolecules That Regulate Cellular Activity and Synaptic Neuronal Control of Specific Tissue Functional Properties.Int J Mol Sci. 2025 Mar 12;26(6):2554. doi: 10.3390/ijms26062554. Int J Mol Sci. 2025. PMID: 40141196 Free PMC article. Review.
-
Mechanisms of breast cancer metastasis: the role of extracellular matrix.Mol Cell Biochem. 2025 May;480(5):2771-2796. doi: 10.1007/s11010-024-05175-x. Epub 2024 Dec 9. Mol Cell Biochem. 2025. PMID: 39652293 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

