Significance of Necroptosis in Cartilage Degeneration
- PMID: 39334958
- PMCID: PMC11429838
- DOI: 10.3390/biom14091192
Significance of Necroptosis in Cartilage Degeneration
Abstract
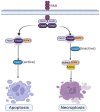
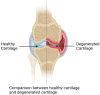
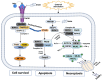
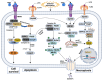
Cartilage, a critical tissue for joint function, often degenerates due to osteoarthritis (OA), rheumatoid arthritis (RA), and trauma. Recent research underscores necroptosis, a regulated form of necrosis, as a key player in cartilage degradation. Unlike apoptosis, necroptosis triggers robust inflammatory responses, exacerbating tissue damage. Key mediators such as receptor-interacting serine/threonine-protein kinase-1 (RIPK1), receptor-interacting serine/threonine-protein kinase-3(RIPK3), and mixed lineage kinase domain-like (MLKL) are pivotal in this process. Studies reveal necroptosis contributes significantly to OA and RA pathophysiology, where elevated RIPK3 and associated proteins drive cartilage degradation. Targeting necroptotic pathways shows promise; inhibitors like Necrostatin-1 (Nec-1), GSK'872, and Necrosulfonamide (NSA) reduce necroptotic cell death, offering potential therapeutic avenues. Additionally, autophagy's role in mitigating necroptosis-induced damage highlights the need for comprehensive strategies addressing multiple pathways. Despite these insights, further research is essential to fully understand necroptosis' mechanisms and develop effective treatments. This review synthesizes current knowledge on necroptosis in cartilage degeneration, aiming to inform novel therapeutic approaches for OA, RA, and trauma.
Keywords: OA; RA; cartilage degeneration; necroptosis; trauma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Marc C.H., editor. Rheumatoid Arthritis. Mosby; Philadelphia, PA, USA: 2009. CHAPTER 8G—Chondrocytes: Pathogenesis of Rheumatoid Arthritis; pp. 151–162.
-
- Buckwalter J.A., Mankin H.J., Grodzinsky A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. -Am. Acad. Orthop. Surg. 2005;54:465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

