Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review
- PMID: 39334976
- PMCID: PMC11428266
- DOI: 10.3390/antibiotics13090801
Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review
Abstract
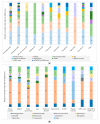
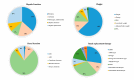
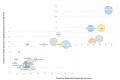
(1) Background: Knowledge about the behavior of antibiotics in critically ill patients has been increasing in recent years. Some studies have concluded that a high percentage may be outside the therapeutic range. The most likely cause of this is the pharmacokinetic variability of critically ill patients, but it is not clear which factors have the greatest impact. The aim of this systematic review is to identify risk factors among critically ill patients that may exhibit significant pharmacokinetic alterations, compromising treatment efficacy and safety. (2) Methods: The search included the PubMed, Web of Science, and Embase databases. (3) Results: We identified 246 observational studies and ten clinical trials. The most studied risk factors in the literature were renal function, weight, age, sex, and renal replacement therapy. Risk factors with the greatest impact included renal function, weight, renal replacement therapy, age, protein or albumin levels, and APACHE or SAPS scores. (4) Conclusions: The review allows us to identify which critically ill patients are at a higher risk of not reaching therapeutic targets and helps us to recognize the extensive number of risk factors that have been studied, guiding their inclusion in future studies. It is essential to continue researching, especially in real clinical practice and with clinical outcomes.
Keywords: antibiotic; critically ill patients; exposure; pharmacodynamics; pharmacokinetics; risk factors; target attainment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. - DOI - PMC - PubMed
-
- Blasco A.C., Alfaro L.A., Reinoso J.C., Mestre M.J.G., Rodríguez-Gascón A. Análisis Farmacocinético-Farmacodinámico en Microbiología: Herramienta para Evaluar el Tratamiento Antimicrobiano Enfermedades Infecciosas y Microbiologia Clinica. Volume 33. Elsevier Doyma; Amsterdam, The Netherlands: 2015. pp. 48–57. - PubMed
-
- Rizk M.L., Bhavnani S.M., Drusano G., Dane A., Eakin A.E., Guina T., Jang S.H., Tomayko J.F., Wang J., Zhuang L., et al. Considerations for Dose Selection and Clinical Pharmacokinetics/Pharmacodynamics for the Development of Antibacterial Agents. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02309-18. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

