Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance
- PMID: 39335035
- PMCID: PMC11429240
- DOI: 10.3390/antibiotics13090862
Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance
Abstract
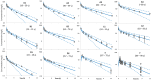
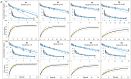
The impact of physiological changes during aging on drug disposition has not always been thoroughly assessed in clinical studies. This has left an open question such as how and to what extent patho- and physiological changes in renal function can affect pharmacokinetics in the geriatric population. The objective of this work was to use a physiologically based pharmacokinetic (PBPK) model to quantify the impact of aging and renal impairment (RI) separately and together on ceftazidime pharmacokinetics (PK). The predicted plasma concentrations and PK parameters from the PBPK model were compared to the observed data in individuals of different ages with or without RI (16 independent studies were investigated in this analysis). Apart from clearance in one study, the predicted ceftazidime PK parameters of young adults, elderly, and in individuals with different levels of renal function were within 2-fold of the observed data, and the observed concentrations fell within the 5th-95th prediction interval from the PBPK model simulations. The PBPK model predicted a 1.2-, 1.5-, and 1.8-fold increase in the plasma exposure (AUC) ratio in individuals aged 40, 60, and 70 years old, respectively, with normal renal function for their age compared to 20-year-old individuals with normal renal function. The impact of RI on ceftazidime was predicted to be less marked in older individuals (a 1.04-, 1.43-, and 2.55-fold change in mild, moderate, or severe RI compared to a healthy age-matched control) than in younger individuals (where a 1.47-, 2.03-, and 3.50-fold increase was predicted in mild, moderate, or severe RI compared to a healthy age-matched control). Utilization of the applied population-based PBPK approach allows delineation of the effects of age from renal disease and can better inform future study design and dosing recommendations in clinical study of elderly patients depending on their age and renal function.
Keywords: GFR; PBPK model; ceftazidime; elderly; renal; renal impairment.
Conflict of interest statement
All authors are paid employees of Certara UK Limited (Certara Predictive Technologies Division) and may hold shares in Certara. The authors indicate no other conflicts of interest.
Figures


References
-
- United Nations. Population Divisions World Population Ageing 2017—Highlights. United Nations, Department of Economic and Social Affairs. 2017. [(accessed on 24 January 2024)]. Available online: https://www.un.org/en/development/desa/population/publications/pdf/agein....
LinkOut - more resources
Full Text Sources

