A Rhein-Based Derivative Targets Staphylococcus aureus
- PMID: 39335055
- PMCID: PMC11428220
- DOI: 10.3390/antibiotics13090882
A Rhein-Based Derivative Targets Staphylococcus aureus
Abstract
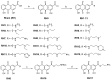
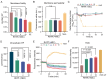
The rise in antibiotic-resistant bacteria highlights the need for novel antimicrobial agents. This study presents the design and synthesis of a series of rhein (RH)-derived compounds with improved antimicrobial properties. The lead compound, RH17, exhibited a potent antibacterial activity against Staphylococcus aureus (S. aureus) isolates, with minimum inhibitory concentrations (MICs) ranging from 8 to 16 μg/mL. RH17 disrupted bacterial membrane stability, hindered metabolic processes, and led to an increase in reactive oxygen species (ROS) production. These mechanisms were confirmed through bacterial growth inhibition assays, membrane function assessments, and ROS detection. Notably, RH17 outperformed the parent compound RH and demonstrated bactericidal effects in S. aureus. The findings suggest that RH17 is a promising candidate for further development as an antimicrobial agent against Gram-positive pathogens, addressing the urgent need for new therapies.
Keywords: Gram-positive pathogen; anthraquinone derivatives; membrane target; rhein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- World Health Organization . Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2018.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

