Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus aureus-Infected Wound Healing
- PMID: 39335062
- PMCID: PMC11428637
- DOI: 10.3390/antibiotics13090889
Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus aureus-Infected Wound Healing
Abstract
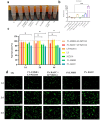
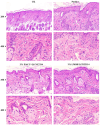
Wound infections caused by Staphylococcus aureus often result in localized suppurative lesions that severely impede the healing process, so it is urgent to develop a dress with efficient antimicrobial and pro-healing functions. In this study, the bifunctional injectable hydrogel lactoferrin (Lf)/NZ2114/lithium magnesium silicate hydrogel (LMSH) was first successfully prepared through the electrostatic interaction method. The physical, biological, and efficacy properties are systematically analyzed with good shear-thinning capacity and biocompatibility. More importantly, it inhibits infection and promotes wound healing in a mouse wound infection model after 14 d treatment, and the bactericidal rate and healing rate were over 99.92% and nearly 100%, respectively. Meanwhile, the massive reduction of inflammatory cells, restoration of tissue structure, and angiogenesis in mice showed the anti-inflammatory and pro-healing properties of the hydrogel. The healed wounds showed thickening with more hair follicles and glands, suggesting that the hydrogel Lf/NZ2114/LMSH (Three in One) could be a better dressing candidate for the treatment of S. aureus-induced wound infections.
Keywords: Lf/NZ2114/LMSH (Three in One); Staphylococcus aureus infection; injectable hydrogel; would dressing.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Roy S., Santra S., Das A., Dixith S., Sinha M., Ghatak S., Ghosh N., Banerjee P., Khanna S., Mathew-Steiner S. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2020;271:1174–1185. doi: 10.1097/SLA.0000000000003053. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

