Antibacterial and Antibiofilm Potential of Chlorophyllin Against Streptococcus mutans In Vitro and In Silico
- PMID: 39335072
- PMCID: PMC11428499
- DOI: 10.3390/antibiotics13090899
Antibacterial and Antibiofilm Potential of Chlorophyllin Against Streptococcus mutans In Vitro and In Silico
Abstract
Background: Streptococcus mutans is a leading causative agent of dental caries and exerts pathogenicity by forming biofilms. Dental caries continues to be a significant public health issue worldwide, affecting an estimated 2.5 billion people, showing a 14.6% increase over the past decade. Herein, the antibacterial potential of Chlorophyllin extracted from Spinacia oleracea was evaluated against biofilm-forming S. mutans via in vitro and in silico studies.
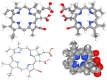
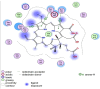
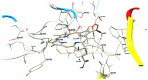
Methodology: The antimicrobial activity of chlorophyllin extract against S. mutans isolates was tested using the agar well diffusion method. Chlorophyllin extract was also tested against biofilm-forming isolates of S. mutans. Chlorophyllin was docked with the antigen I/II (AgI/II) protein of S. mutans to evaluate its antimicrobial mechanism. The chemical structure and canonical SMILES format of Chlorophyllin were obtained from PubChem. Additionally, adsorption, distribution, metabolism, excretion, and toxicity (ADMET) analyses of Chlorophyllin were performed using ADMETlab 2.0 to assess its pharmacokinetic properties.
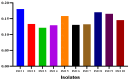
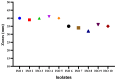
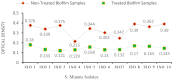
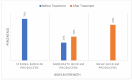
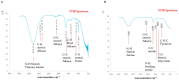
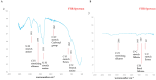
Results: An agar well diffusion assay revealed that all S. mutans isolates were susceptible to Chlorophyllin extract and showed a variety of inhibition zones ranging from 32 to 41 mm. Chlorophyllin reduces the biofilm strength of four isolates from strong to moderate and six from strong to weak. The antibiofilm potential of Chlorophyllin was measured by a reduction in the number of functional groups observed in the Fourier Transform Infrared Spectrometer (FTIR) spectra of the extracellular polymeric substance (EPS) samples. Chlorophyllin showed binding with AgI/II proteins of S. mutans, which are involved in adherence to the tooth surface and initiating biofilm formation. The ADMET analysis revealed that the safety of Chlorophyllin exhibited favorable pharmacokinetic properties.
Conclusions: Chlorophyllin stands out as a promising antibacterial and antibiofilm agent against biofilm-forming S. mutans, and its safety profile highlights its potential suitability for further investigation as a therapeutic agent.
Keywords: ADMET analysis; Streptococcus mutans; antibacterial activity; biofilms; chlorophyllin; dental caries; proteins docking.
Conflict of interest statement
No conflicts of interest exist with regard to the work described.
Figures

References
-
- Tang X., Kudo Y., Baker J.L., Labonte S., Jordan P.A., McKinnie S.M.K., Guo J., Huan T., Moore B.S., Edlund A. Cariogenic Streptococcus mutans Produces Tetramic Acid Strain-Specific Antibiotics That Impair Commensal Colonization. ACS Infect. Dis. 2020;6:563–571. doi: 10.1021/acsinfecdis.9b00365. - DOI - PMC - PubMed
-
- Kassebaum N.J., Smith A.G.C., Bernabé E., Fleming T.D., Reynolds A.E., Vos T., Murray C.J.L., Marcenes W., Abyu G.Y., Alsharif U., et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017;96:380–387. doi: 10.1177/0022034517693566. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

