Clarithromycin Modulates Neutrophilic Inflammation Induced by Prevotella intermedia in Human Airway Epithelial Cells
- PMID: 39335081
- PMCID: PMC11429484
- DOI: 10.3390/antibiotics13090909
Clarithromycin Modulates Neutrophilic Inflammation Induced by Prevotella intermedia in Human Airway Epithelial Cells
Abstract
Objectives: In the present study, we aimed to clarify the mechanisms by which periodontal pathogens, particularly Prevotella intermedia, induce severe neutrophilic inflammation. In addition, we aimed to test the efficacy of macrolides, which has not been resolved in the neutrophilic inflammation induced by P. intermedia. Methods: NCl-H292 human airway epithelial cells were pre-incubated with clarithromycin for 2 h before incubation with P. intermedia supernatants. Then, C-X-C motif chemokine ligand 8 (CXCL8) transcription and interleukin (IL)-8 production were measured. To elucidate the signaling pathway, mitogen-activated protein kinase inhibitors were added to the cell culture, and the cells were subjected to Western blotting. Results:P. intermedia supernatants promoted CXCL8 transcription and IL-8 production, and the reactions were significantly suppressed by clarithromycin pretreatment. Only trametinib, the selective mitogen-activated extracellular signal-regulated kinase inhibitor, downregulated CXCL8 transcription and IL-8 production. Furthermore, Western blotting revealed that stimulation with P. intermedia supernatants specifically induces extracellular signal-regulated kinases (ERK) 1/2 phosphorylation, which is suppressed by clarithromycin pretreatment. Notably, the interference analysis revealed that ERK3 might be dispensable for IL-8 production under the stimulation of P. intermedia supernatants. Conclusions: Our results provide new insight into the mechanism underlying P. intermedia-induced production of IL-8 from human airway epithelial cells. Furthermore, macrolides might have therapeutic potential in regulating periodontal pathogen-induced neutrophilic inflammation in the lungs.
Keywords: Prevotella intermedia; clarithromycin; human airway epithelial cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



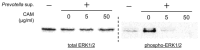

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

