Feasibility Study of Nivolumab in Combination with Carboplatin Plus Paclitaxel and Concurrent Thoracic Radiation in Patients with Untreated Unresectable Locally Advanced Non-Small Cell Lung Cancer
- PMID: 39335099
- PMCID: PMC11430718
- DOI: 10.3390/cancers16183127
Feasibility Study of Nivolumab in Combination with Carboplatin Plus Paclitaxel and Concurrent Thoracic Radiation in Patients with Untreated Unresectable Locally Advanced Non-Small Cell Lung Cancer
Abstract
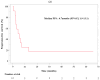
Despite advancements in diagnosing and treating non-small cell lung cancer (NSCLC), the prognosis remains poor. Immune checkpoint inhibitors have shown promise in enhancing survival rates. Therefore, this study aimed to investigate the safety of nivolumab administration with concurrent chemoradiation therapy (CCRT) in patients with unresectable locally advanced NSCLC. Twelve patients with unresectable locally advanced NSCLC at Kansai Medical University Hospital and Izumi City General Medical Center were enrolled from May 2018 to September 2020. They received nivolumab (360 mg) tri-weekly twice, weekly carboplatin (AUC 2 min × mg/mL) and paclitaxel (40 mg/m2) for 6 weeks, and thoracic radiotherapy (60 Gy/30 fractions), followed by maintenance nivolumab therapy (360 mg, tri-weekly) for 6 months. The primary endpoint was incidence of dose-limiting toxicities (DLTs), and the secondary endpoints included safety, response rate, progression-free survival (PFS), overall survival (OS), 2-year survival rate, and treatment completion rate. Three patients completed the protocol. Nine discontinued due directly to interstitial pneumonia (three) and pneumonia (one). Ten patients (83.3%) experienced a grade 3 or higher event, of which three (25%) experienced a grade 4 or higher event, and of these, one (8.3%) experienced a grade 5 event. Three patients experienced DLTs. Concurrent nivolumab with CCRT was tolerated in unresectable locally advanced NSCLC, which offers potential treatment benefits.
Keywords: chemoradiotherapy; feasibility study; nivolumab; unresectable locally advanced non-small cell lung cancer.
Conflict of interest statement
Takayasu Kurata received grants from MSD, Astra Zeneca, Amgen, Boehringer Ingelheim, Daiichi Sankyo Pharmaceutical, Takeda Pharmaceutical, and Bristol Myers Squibb, and honoraria for a lecture from Astra Zeneca, Ono Pharmaceutical, MSD, Nippon Kayaku, Takeda Pharmaceutical, Eli Lilly, Bristol Myers Squibb, Chugai Pharmaceutical, and Pfizer. Hiroshige Yoshioka received honoraria for lecture fees from Boehringer Ingelheim, Chugai Pharmaceutical, Nippon Kayaku, Taiho Pharmaceutical, Eli Lilly, Takeda Pharmaceutical, and Bristol Myers Squibb. The other authors declare no conflicts of interest.
Figures


References
-
- Curran W.J., Jr., Paulus R., Langer C.J., Komaki R., Lee J.S., Hauser S., Movsas B., Wasserman T., Rosenthal S.A., Gore E., et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. - DOI - PMC - PubMed
-
- Yamamoto N., Nakagawa K., Nishimura Y., Tsujino K., Satouchi M., Kudo S., Hida T., Kawahara M., Takeda K., Katakami N., et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. - DOI - PubMed
-
- Segawa Y., Kiura K., Takigawa N., Kamei H., Harita S., Hiraki S., Watanabe Y., Sugimoto K., Shibayama T., Yonei T., et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J. Clin. Oncol. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. - DOI - PubMed
-
- Sause W.T., Scott C., Taylor S., Johnson D., Livingston R., Komaki R., Emami B., Curran W.J., Byhardt R.W., Turrisi A.T. Radiation Therapy Oncology Group (RTOG) 88–08 and Eastern Cooperative Oncology Group (ECOG) 4588: Preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J. Natl. Cancer Inst. 1995;87:198–205. doi: 10.1093/jnci/87.3.198. - DOI - PubMed
-
- Furuse K., Fukuoka M., Kawahara M., Nishikawa H., Takada Y., Kudoh S., Katagami N., Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

