GABA(A) Receptor Activation Drives GABARAP-Nix Mediated Autophagy to Radiation-Sensitize Primary and Brain-Metastatic Lung Adenocarcinoma Tumors
- PMID: 39335139
- PMCID: PMC11430345
- DOI: 10.3390/cancers16183167
GABA(A) Receptor Activation Drives GABARAP-Nix Mediated Autophagy to Radiation-Sensitize Primary and Brain-Metastatic Lung Adenocarcinoma Tumors
Abstract
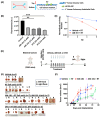
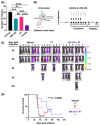
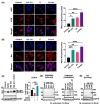
In non-small cell lung cancer (NSCLC) treatment, radiotherapy responses are not durable and toxicity limits therapy. We find that AM-101, a synthetic benzodiazepine activator of GABA(A) receptor, impairs the viability and clonogenicity of both primary and brain-metastatic NSCLC cells. Employing a human-relevant ex vivo 'chip', AM-101 is as efficacious as docetaxel, a chemotherapeutic used with radiotherapy for advanced-stage NSCLC. In vivo, AM-101 potentiates radiation, including conferring a significant survival benefit to mice bearing NSCLC intracranial tumors generated using a patient-derived metastatic line. GABA(A) receptor activation stimulates a selective-autophagic response via the multimerization of GABA(A) receptor-associated protein, GABARAP, the stabilization of mitochondrial receptor Nix, and the utilization of ubiquitin-binding protein p62. A high-affinity peptide disrupting Nix binding to GABARAP inhibits AM-101 cytotoxicity. This supports a model of GABA(A) receptor activation driving a GABARAP-Nix multimerization axis that triggers autophagy. In patients receiving radiotherapy, GABA(A) receptor activation may improve tumor control while allowing radiation dose de-intensification to reduce toxicity.
Keywords: GABA receptor; GABARAP; Nix; autophagy; benzodiazepine; brain metastasis; lung cancer; p62; radiotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Update of
-
GABA(A) receptor activation drives GABARAP-Nix mediated autophagy to radiation-sensitize primary and brain-metastatic lung adenocarcinoma tumors.bioRxiv [Preprint]. 2023 Dec 1:2023.11.29.569295. doi: 10.1101/2023.11.29.569295. bioRxiv. 2023. Update in: Cancers (Basel). 2024 Sep 15;16(18):3167. doi: 10.3390/cancers16183167. PMID: 38076805 Free PMC article. Updated. Preprint.
References
-
- Fuchs J., Fruh M., Papachristofilou A., Bubendorf L., Hauptle P., Jost L., Zippelius A., Rothschild S.I. Resection of isolated brain metastases in non-small cell lung cancer (NSCLC) patients—Evaluation of outcome and prognostic factors: A retrospective multicenter study. PLoS ONE. 2021;16:e0253601. doi: 10.1371/journal.pone.0253601. - DOI - PMC - PubMed
-
- Baschnagel A.M., Elnaggar J.H., VanBeek H.J., Kromke A.C., Skiba J.H., Kaushik S., Abel L., Clark P.A., Longhurst C.A., Nickel K.P., et al. ATR inhibitor M6620 (VX-970) enhances the effect of radiation in non-small cell lung cancer brain metastasis patient-derived xenografts. Mol. Cancer Ther. 2021;20:2129–2139. doi: 10.1158/1535-7163.MCT-21-0305. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

