Combination of JAKi and HDACi Exerts Antiangiogenic Potential in Cutaneous T-Cell Lymphoma
- PMID: 39335148
- PMCID: PMC11430229
- DOI: 10.3390/cancers16183176
Combination of JAKi and HDACi Exerts Antiangiogenic Potential in Cutaneous T-Cell Lymphoma
Abstract
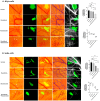
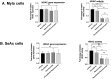
Angiogenesis plays a pivotal role in the growth and metastasis of tumors, including the development and progression of cutaneous lymphomas. The chick embryo CAM model has been utilized in various studies to assess the growth rate, angiogenic potential, and metastatic capability of different tumor types and malignant cell lines. However, the precise mechanisms of angiogenesis in CTCL and the influence of Ruxolitinib or Resminostat on angiogenesis in hematological malignancies and solid tumors are not well understood. Recent in vitro and in vivo data have demonstrated the synergistic inhibition of tumorigenesis and metastasis in experimental models of CTCL when using the combination of Resminostat (HDACi) with Ruxolitinib (JAKi). The present work aims to elucidate the effects of this combination on the tumor microenvironment's vascular components. We investigated the effects of Ruxolitinib (JAKi) in combination with Resminostat (HDACi) treatment in transendothelial migration of CTCL cells (106 MyLa and SeAx) by using a transwell-based transendothelial migration assay and tumor angiogenesis in vivo. We used the CTCL chick embryo CAM model with xenografted tumors derived from implanted MyLa and SeAx cells and administered topically 15 μM ruxolitinib and 5 μM Resminostat every two days during a 5-day period. JAKi and HDACi inhibited CTCL cell transendothelial migration by 75% and 82% (p < 0.05) in both CTCL engrafted cells (MyLa and SeAx, respectively) compared to the untreated group. Moreover, the combination of ruxolitinib with resminostat blocked angiogenesis by significantly reducing the number of blood vessel formation by 49% and 34% in both MyLa and SeAx, respectively (p < 0.05), indicating that the proposed combination exerted significant anti-angiogenic effects in the CAM CTCL model. Overall, these data provide valuable insights into potential therapeutic strategies targeting angiogenesis in CTCL, paving the way for more effective treatment approaches in the future.
Keywords: CTCL; angiogenesis; chick embryo model; resminostat; ruxolitinib.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Scarisbrick J.J., Hodak E., Bagot M., Stranzenbach R., Stadler R., Ortiz-Romero P.L., Papadavid E., Evison F., Knobler R., Quaglino P., et al. Blood Classification and Blood Response Criteria in Mycosis Fungoides and Sézary Syndrome Using Flow Cytometry: Recommendations from the EORTC Cutaneous Lymphoma Task Force. Eur. J. Cancer. 2018;93:47–56. doi: 10.1016/J.EJCA.2018.01.076. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

