Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine?
- PMID: 39335186
- PMCID: PMC11429908
- DOI: 10.3390/cancers16183216
Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine?
Abstract
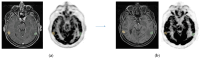
Brain metastases or primary brain tumours had poor prognosis until the use of high dose radiotherapy. However, radionecrosis is a complex challenge in the post-radiotherapy management of these patients due to the difficulty of distinguishing this complication from local tumour recurrence. MRI alone has a variable specificity and sensibility, as does PET-CT imaging. We aimed to investigate the diagnostic performance of dual-phase 18F-FDG PET-mpMRI to distinguish cerebral radionecrosis from local tumour recurrence after cranial radiotherapy. A retrospective analysis was conducted between May 2021 and September 2022. Inclusion criteria encompassed patients with inconclusive MRI findings post-radiotherapy and history of cerebral radiotherapy for primary or metastatic brain lesions. Lesions are assessed qualitatively and semi-quantitatively. The gold standard to assess radionecrosis was histopathology or a composite criterion at three months. The study evaluated 24 lesions in 23 patients. Qualitative analysis yielded 85.7% sensitivity and 75% specificity. Semi-quantitative analysis, based on contralateral background noise, achieved 100% sensitivity and 50% specificity. Moreover, using contralateral frontal lobe background noise resulted in higher performances with 92% sensitivity and 63% specificity. Stratification by lesion type demonstrated 100% sensitivity and specificity rates for metastatic lesions. The diagnostic performance of dual-phase 18F-FDG PET-mpMRI shows promising results for metastatic lesions.
Keywords: PET-MRI; brain radionecrosis; cerebral tumour recurrence; cranial radiotherapy; dual-phase FDG.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bodensohn R., Kaempfel A.-L., Boulesteix A.-L., Orzelek A.M., Corradini S., Fleischmann D.F., Forbrig R., Garny S., Hadi I., Hofmaier J., et al. Stereotactic Radiosurgery versus Whole-Brain Radiotherapy in Patients with 4–10 Brain Metastases: A Nonrandomized Controlled Trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2023;186:109744. doi: 10.1016/j.radonc.2023.109744. - DOI - PubMed
-
- Gondi V., Bauman G., Bradfield L., Burri S.H., Cabrera A.R., Cunningham D.A., Eaton B.R., Hattangadi-Gluth J.A., Kim M.M., Kotecha R., et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022;12:265–282. doi: 10.1016/j.prro.2022.02.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

