Utility of Quantitative EEG in Neurological Emergencies and ICU Clinical Practice
- PMID: 39335433
- PMCID: PMC11430096
- DOI: 10.3390/brainsci14090939
Utility of Quantitative EEG in Neurological Emergencies and ICU Clinical Practice
Abstract
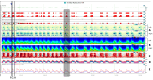
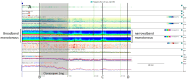
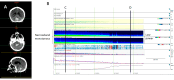
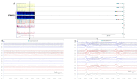
The electroencephalogram (EEG) is a cornerstone tool for the diagnosis, management, and prognosis of selected patient populations. EEGs offer significant advantages such as high temporal resolution, real-time cortical function assessment, and bedside usability. The quantitative EEG (qEEG) added the possibility of long recordings being processed in a compressive manner, making EEG revision more efficient for experienced users, and more friendly for new ones. Recent advancements in commercially available software, such as Persyst, have significantly expanded and facilitated the use of qEEGs, marking the beginning of a new era in its application. As a result, there has been a notable increase in the practical, real-world utilization of qEEGs in recent years. This paper aims to provide an overview of the current applications of qEEGs in daily neurological emergencies and ICU practice, and some elementary principles of qEEGs using Persyst software in clinical settings. This article illustrates basic qEEG patterns encountered in critical care and adopts the new terminology proposed for spectrogram reporting.
Keywords: cyclic patterns; frequency domain; qEEG; rhythmic patterns; seizures; spectral analysis; spectrogram; time domain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- Berger H. Über das Elektrenkephalogramm des Menschen. Arch. Für. Psychiatry Nervenkrankh. 1929;87:527–570. doi: 10.1007/BF01797193. - DOI
-
- Tatum W., Rubboli G., Kaplan P., Mirsatari S., Radhakrishnan K., Gloss D., Caboclo L., Drislane F., Koutroumanidis M., Schomer D., et al. Clinical utility of EEG in diagnosing and monitoring epilepsy in adults. Clin. Neurophysiol. 2018;129:1056–1082. doi: 10.1016/j.clinph.2018.01.019. - DOI - PubMed
-
- Leitinger M., Gaspard N., Hirsch L.J., Beniczky S., Kaplan P.W., Husari K., Trinka E. Diagnosing nonconvulsive status epilepticus: Defining electroencephalographic and clinical response to diagnostic intravenous antiseizure medication trials. Epilepsia. 2023;64:2351–2360. doi: 10.1111/epi.17694. - DOI - PubMed
-
- Hirsch L.J., Fong M.W.K., Brenner R.P. Hirsch and Brenner’s Atlas of EEG in Critical Care. 2nd ed. John Wiley & Sons; Oxford, UK: 2023.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

