Postoperative Atrial Fibrillation: A Review
- PMID: 39335482
- PMCID: PMC11428825
- DOI: 10.3390/biomedicines12091968
Postoperative Atrial Fibrillation: A Review
Abstract
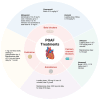
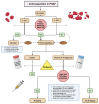
Atrial fibrillation (AF) in the postoperative phase is a manifestation of numerous factors, including surgical stress, anesthetic effects, and underlying cardiovascular conditions. The resultant cardiac hyperactivity can induce new onset or exacerbate existing AF. A common phenomenon, postoperative atrial fibrillation (POAF) affects nearly 40% of patients and is associated with longer hospitalization stays, and increased mortality, heart failure, stroke, and healthcare costs. Areas of controversy in POAF include whether to anticoagulate patients who have short-lived POAF, especially given their higher bleeding risk in the postoperative period, and the identification of patients who would benefit the most from preventive drug therapy for POAF. This review discusses the pathophysiology and management of POAF, and strategies to reduce its occurrence.
Keywords: anticoagulation; atrial fibrillation; bleeding risk; direct-acting anticoagulants; perioperative; stroke; warfarin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Egbe A.C., Miranda W.R., Anderson J.H., DeSimone C.V., Andi K., Goda A.Y., Stephens E.H., Dearani J.A., Crestanello J., Connolly H.M., et al. Outcome of New-Onset Postoperative Atrial Fibrillation after Cardiac Surgery in Adults with Congenital Heart Disease. JACC Clin. Electrophysiol. 2022;8:1407–1416. doi: 10.1016/j.jacep.2022.08.033. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

