Investigation of High Frequency Irreversible Electroporation for Canine Spontaneous Primary Lung Tumor Ablation
- PMID: 39335552
- PMCID: PMC11428908
- DOI: 10.3390/biomedicines12092038
Investigation of High Frequency Irreversible Electroporation for Canine Spontaneous Primary Lung Tumor Ablation
Abstract
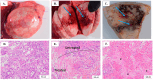
In this study, the feasibility of treating canine primary lung tumors with high-frequency irreversible electroporation (H-FIRE) was investigated as a novel lung cancer treatment option. H-FIRE is a minimally invasive tissue ablation modality that delivers bipolar pulsed electric fields to targeted cells, generating nanopores in cell membranes and rendering targeted cells nonviable. In the current study, canine patients (n = 5) with primary lung tumors underwent H-FIRE treatment with an applied voltage of 2250 V using a 2-5-2 µs H-FIRE waveform to achieve partial tumor ablation prior to the surgical resection of the primary tumor. Surgically resected tumor samples were evaluated histologically for tumor ablation, and with immunohistochemical (IHC) staining to identify cell death (activated caspase-3) and macrophages (IBA-1, CD206, and iNOS). Changes in immunity and inflammatory gene signatures were also evaluated in tumor samples. H-FIRE ablation was evident by the microscopic observation of discrete foci of acute hemorrhage and necrosis, and in a subset of tumors (n = 2), we observed a greater intensity of cleaved caspase-3 staining in tumor cells within treated tumor regions compared to adjacent untreated tumor tissue. At the study evaluation timepoint of 2 h post H-FIRE, we observed differential gene expression changes in the genes IDO1, IL6, TNF, CD209, and FOXP3 in treated tumor regions relative to paired untreated tumor regions. Additionally, we preliminarily evaluated the technical feasibility of delivering H-FIRE percutaneously under CT guidance to canine lung tumor patients (n = 2). Overall, H-FIRE treatment was well tolerated with no adverse clinical events, and our results suggest H-FIRE potentially altered the tumor immune microenvironment.
Keywords: canine oncology; comparative oncology; lung cancer; tumor ablation.
Conflict of interest statement
The authors declare the following conflict of interest: The author R.V.D. has ownership interest in the startup companies within the field of bioelectrics. R.V.D. also receives royalty income from technologies he has invented and serves as a consultant. Authors Lorenzo, Aycock, and Davalos have patents in the area of electroporation.
Figures





References
-
- McPhetridge J.B., Scharf V.F., Regier P.J., Toth D., Lorange M., Tremolada G., Dornbusch J.A., Selmic L.E., Bae S., Townsend K.L., et al. Distribution of histopathologic types of primary pulmonary neoplasia in dogs and outcome of affected dogs: 340 cases (2010–2019) J. Am. Vet. Med. Assoc. 2021;260:234–243. doi: 10.2460/javma.20.12.0698. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

