STAT3 Protein-Protein Interaction Analysis Finds P300 as a Regulator of STAT3 and Histone 3 Lysine 27 Acetylation in Pericytes
- PMID: 39335615
- PMCID: PMC11428717
- DOI: 10.3390/biomedicines12092102
STAT3 Protein-Protein Interaction Analysis Finds P300 as a Regulator of STAT3 and Histone 3 Lysine 27 Acetylation in Pericytes
Abstract
Background: Signal transducer and activator of transcription 3 (STAT3) is a member of the cytoplasmic inducible transcription factors and plays an important role in mediating signals from cytokines, chemokines, and growth factors. We and others have found that STAT3 directly regulates pro-fibrotic signaling in the kidney. The STAT3 protein-protein interaction plays an important role in activating its transcriptional activity. It is necessary to identify these interactions to investigate their function in kidney disease. Here, we investigated the protein-protein interaction among three species to find crucial interactions that can be targeted to alleviate kidney disease.
Method: In this study, we examined common protein-protein interactions leading to the activation or downregulation of STAT3 among three different species: humans (Homo sapiens), mice (Mus musculus), and rabbits (Oryctolagus cuniculus). Further, we chose to investigate the P300 and STAT3 interaction and performed studies of the activation of STAT3 using IL-6 and inhibition of the P300 by its specific inhibitor A-485 in pericytes. Next, we performed immunoprecipitation to confirm whether A-485 inhibits the binding of P300 to STAT3.
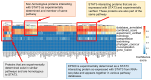
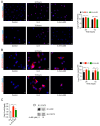
Results: Using the STRING application from ExPASy, we found that six proteins, including PIAS3, JAK1, JAK2, EGFR, SRC, and EP300, showed highly confident interactions with STAT3 in humans, mice, and rabbits. We also found that IL-6 treatment increased the acetylation of STAT3 and increased histone 3 lysine acetylation (H3K27ac). Furthermore, we found that the disruption of STAT3 and P300 interaction by the P300 inhibitor A-485 decreased STAT3 acetylation and H3K27ac. Finally, we confirmed that the P300 inhibitor A-485 inhibited the binding of STAT3 with P300, which inhibited its transcriptional activity by reducing the expression of Ccnd1 (Cyclin D1).
Conclusions: Targeting the P300 protein interaction with STAT3 may alleviate STAT3-mediated fibrotic signaling in humans and other species.
Keywords: EGFR; EP300; PIAS3; STAT3 acetylation; STRING; histone acetylation; pericytes; protein–protein interaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Zhou Y.-B., Zhou H., Li L., Kang Y., Cao X., Wu Z.-Y., Ding L., Sethi G., Bian J.-S. Hydrogen sulfide prevents elastin loss and attenuates calcification induced by high glucose in smooth muscle cells through suppression of Stat3/Cathepsin S signaling pathway. Int. J. Mol. Sci. 2019;20:4202. doi: 10.3390/ijms20174202. - DOI - PMC - PubMed
-
- Shanmugam M.K., Lee J.H., Chai E.Z.P., Kanchi M.M., Kar S., Arfuso F., Dharmarajan A., Kumar A.P., Ramar P.S., Looi C.Y. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016;40–41:35–47. doi: 10.1016/j.semcancer.2016.03.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

