Exploring the Antibacterial and Regenerative Properties of a Two-Stage Alginate Wound Dressing in a Rat Model of Purulent Wounds
- PMID: 39335635
- PMCID: PMC11430427
- DOI: 10.3390/biomedicines12092122
Exploring the Antibacterial and Regenerative Properties of a Two-Stage Alginate Wound Dressing in a Rat Model of Purulent Wounds
Abstract
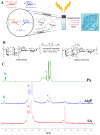
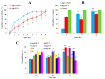
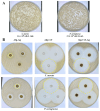
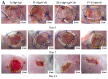
Chronic wounds complicated by infection pose significant clinical challenges, necessitating comprehensive treatment approaches. The widespread use of antibiotics has led to resistant microorganisms, complicating traditional therapies. This study aims to develop and evaluate modified alginate wound dressings with enhanced antimicrobial and regenerative properties. Alginate dressings were synthesized with silver nanoparticles, cefepime, and fibroblast growth factor-2 (FGF-2). The two-stage therapy involved an initial antibacterial dressing followed by a regenerative dressing. In vitro tests demonstrated high antibacterial activity, with maximum inhibition zones for P. aeruginosa (41.3 ± 0.4 mm) and S. aureus (36.6 ± 1.8 mm). In vivo studies on rats with purulent wounds showed significant healing progression in the experimental group. Histological analysis revealed complete re-epithelialization, thicker neoepithelium, dense collagen deposition, and minimal inflammation in treated wounds. These findings suggest that the modified alginate dressings significantly enhance the reparative process and are promising for treating chronic infected wounds in both veterinary and medical practices.
Keywords: FGF-2; alginate wound dressings; antibacterial properties; cefepime; chronic wounds; regenerative medicine; silver nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Shmatenko O.P., Pidlisny O.V., Prykhodko T.V., Solomennyi A.M., Pritula R.L., Semenchenko G.B., Takhtaulova N.O. Technological aspects of creating soft dosage forms for the treatment of purulent wounds. Ukr. J. Mil. Med. 2020;1:50–63. doi: 10.46847/ujmm.2020.1(1)-050. - DOI
-
- Zubarev P.N., Ivanus S.Y., Risman B. V Modern Principles of Treatment of Purulent Wounds. Volume 37 Food and Agriculture Organization of the United Nations; Rome, Italy: 2017.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

